Contents
- What is cefotaxime?
- Cefotaxime molecular formula, weight, structure, IUPAC name and class
- What is the mechanism of action of cefotaxime?
- Pharmacokinetics of cefotaxime
- What are the indications and usage of cefotaxime?
- What is the dosage of cefotaxime?
- Can cefotaxime be given in pregnancy?
- Can cefotaxime be given to nursing mothers?
- What are the contraindications of cefotaxime?
- What are the side effects of cefotaxime?
- What to do if I overdose cefotaxime?
- Can cefotaxime interact with sodium intake?
- Can cefotaxime be taken in patients with renal dysfunction?
- Can cefotaxime be given in patients with colitis?
- Can cefotaxime be given in patients who are on dialysis?
- Can cefotaxime be given in patients with liver disease?
- Can cefotaxime cause seizure disorders?
- Can cefotaxime be given to a patient who received cholera vaccine recently?
- Can cefotaxime be taken with warfarin?
- Can cefotaxime be taken with furosemide?
- Can cefotaxime be taken with amikacin?
- Can cefotaxime be given with chloramphenicol?
- Can cefotaxime be taken with ethyl estradiol?
- Can cefotaxime be taken with typhoid vaccine?
What is cefotaxime?
Cefotaxime is a third-generation cephalosporin antibiotic. Like other third-generation cephalosporins, it has broad spectrum activity against Gram positive and Gram negative bacteria. In most cases, it is considered to be equivalent to ceftriaxone in terms of safety and efficacy.
Cefotaxime is a cephalosporin antibiotic that works by fighting the bacteria in your body. It is used to treat bacterial infections including severe or life-threatening infections. People who have undergone surgery are more prone to get severe infection and cefotaxime is given to them in order to prevent infections.
It can also be used for the purposes which may not be listed in medicine guide. If you are allergic to cefotaxime or antibiotics which are similar to cefotaxime, such antibiotics are cefdinir, cefprozil, cefuroxime, cephalexin and many others.
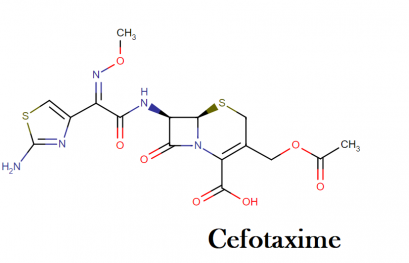
Cefotaxime molecular formula, weight, structure, IUPAC name and class
Molecular formula of cefotaxime: C6H17N5O7S2
Molecular weight: 455.46 g/mol
Molecular structure:
IUPAC name: (6R,7R)-3-(acetyloxymethyl)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
This compound belongs to the class of organic compounds known as cephalosporin 3′-esters. These are cephalosporins that are esterified at the 3′-position.
What is the mechanism of action of cefotaxime?
Cefotaxime is a cephalosporin antibiotic, which is given in the parenteral form. It works by inhibiting the cell wall synthesis of bacteria and exerts its bactericidal action. Cefotaxime is resistant to the action of various enzymes (such as beta-lactamase enzyme), due to its chemical structural modifications.
Cefotaxime demonstrated excellent bactericidal activity against Enterobacteriaceae (gram-negative bacilli), however, it shows less or poor efficacy against Pseudomonas. It shows variable activity against clostridia and Bacteroides species.
Pharmacokinetics of cefotaxime
Cefotaxime is desacetylated to a microbiologically active metabolite known as desacetylated metabolite which is excreted in urine (15%-20%), however, parent compound is excreted in urine (50%-60%). The elimination half-life of cefotaxime is about one hour, with the total body clearance being approximately twice that of the renal clearance.
The elimination half-life of cefotaxime and desacetyl cefotaxime gets increased due to severe renal dysfunction. Most common severe side effects of cefotaxime are local reactions at the injection site and hypersensitivity.
There are very limited reports showing the comparative trials that the cefotaxime is superior to other cephalosporin or aminoglycosides. Due to its excellent activity, cefotaxime is proved most efficacious in combating the serious gram-negative infections and due to its low toxicity profile.
What are the indications and usage of cefotaxime?
Cefotaxime injection is indicated for the treatment of the serious infections caused by susceptible strains of microorganisms. It is mainly indicated for:
- Lower respiratory tract infections
- Genitourinary tract infections
- Gynaecological infections
- Bacteremia/Septicemia
- Skin and skin tissue infections
- Intra-abdominal infections
- Bone or joint infections
- Central nervous system infections
Cefotaxime injection is successfully used in treating the patients with infections caused by susceptible infections caused by susceptible organisms; however, there are many strains of Enterococcus and Pseudomonas species are resistant to cefotaxime.
Cefotaxime injection may be used concomitantly with aminoglycosides in certain cases of confirmed or suspected gram-positive or gram-negative sepsis or in patients with other serious infections in which the causative organism has not been identified. The severity of the infection and patient’s condition describes the dose labeling both the antibiotics.
If the therapy is prolonged or if the higher doses of antibiotics are given, the renal function of the patient should be monitored. The monitoring of the renal function should be done because of potential nephrotoxicity and ototoxicity of aminoglycoside antibiotics. The nephrotoxicity can be increased when cefotaxime is used along with aminoglycosides.
What is the dosage of cefotaxime?
The recommended dose range for cefotaxime in adults is 0.5 grams once a day to 12 grams divided into multiple doses per day.
The recommended dose range for cefotaxime in children includes the following.
- 0 to 1 week of age – 50 mg/kg per dose every 12 hours IV
- 1 to 4 weeks of age – 50 mg/kg per dose every 8 hours IV
- If 1 month to 12 years old:
- Less than 50 kg – 50 to 180 mg/kg IM or IV divided into four to six equal doses
- Greater than or equal to 50 kg – use the usual adult dose (max dose of 12 grams)
You should take cefotaxime as prescribed by your doctor. You should follow the directions given on the prescription label carefully. The cefotaxime dose your doctor recommends will be based on the condition for which you are being treated, other medications that you already taking, any other underlying disease if you have any, how you respond to the medication, your kidney’s function, your weight, and also it depends on your age, Therefore you should discuss all the concerns with your doctor before taking this medication.
Can cefotaxime be given in pregnancy?
There have been no signs of teratogenicity or embryotoxicity observed in animals receiving cefotaxime. No controlled data have been observed in human pregnancy.
This drug comes under pregnancy category B, which stated that, the drugs that were taken by only a limited number of pregnant women and women of childbearing age, and there was no frequency of malformation or other direct or indirect harmful effects on the human fetus were observed. Still, you should talk to your doctor before taking this medication in pregnancy.
Can cefotaxime be given to nursing mothers?
Cefotaxime is excreted in human milk in low concentrations. When cefotaxime injection is administered to nursing mothers, precaution should be taken.
What are the contraindications of cefotaxime?
Cefotaxime is contraindicated in patients who are hypersensitive to cefotaxime or cephalosporin group of antibiotics.
What are the side effects of cefotaxime?
The most commonly reported side effects include injection site inflammation, pain, in duration, and tenderness.
- Local side effects include injection site inflammation, pain, induration, tenderness, postmarketing reports, phlebitis, thrombophlebitis.
- Cefotaxime can also cause some common gastrointestinal side effects colitis, diarrhea, nausea, vomiting; however, pseudomembranous colitis/Clostridium difficile infections that occur during treatment may occur which are rare and are less common.
- Some dermatologic side effects that commonly occur are rashes, skin rashes, and pruritis, uncommon side effects include urticaria, some rare side effects are toxic epidermal necrolysis, Stevens-Johnsons syndrome, erythema multiform. Some postmarketing reports include bullous skin reactions, acute generalized exanthematous pustulosis.
- Here are some hematologic side effects are eosinophilia, uncommon side effects include leucopenia, thrombocytopenia. However, side effects whose frequency not reported are neutropenia, transient leucopenia, granulocytopenia, hemolytic anemia, agranulocytosis, and positive direct Coomb’s tests. The post-marketing hematologic reports of cefotaxime are hemolytic anemia, pancytopenia, bone marrow failure. Thrombocytopenia was also reported in a case but usually was rapidly reversible upon discontinuation.
- Other common side effects are fever, uncommon side effects drug fever. The side effects whose frequency not reported are malaise, superinfection, candidiasis, shivering, systemic reactions to Lidocaine, false-positive test for urinary glucose.
- Some uncommon hepatic side effects are transient AST, bilirubin and alkaline phosphate level elevations. Side effects whose frequency have not been reported are hepatitis and jaundice. Post-marketing hematologic reports of cefotaxime are cholestasis, hepatic dysfunction.
- Cefotaxime usually does not show nervous side effects. However, uncommon side effects are convulsions. A headache, dizziness, encephalopathy, impaired consciousness, abnormal movements are some side effects whose frequency has not been reported. Seizures are side effects of cefotaxime whose frequency has not been reported.
- Some uncommon immunologic side effects of cefotaxime are Jarischh-Herxheimer reaction.
- Cefotaxime can also cause anaphylactic reactions, anaphylactic shock, angioedema, and allergic reactions as hypersensitivity side effects.
- The side effects of cefotaxime also include genitourinary side effects, for example, moniliasis and vaginitis; however, the frequency of such effects have not been reported.
- Similarly, it can also cause transient blood urea nitrogen elevations; but the frequency of such reports has not been reported. Some post-marketing renal reports include interstitial nephritis, transient creatinine elevations, acute renal failure.
- Cefixime can also cause difficulty in breathing as a respiratory side effect but that is also without any reported frequency. As per post-marketing reports, it can cause bronchospasm.
- Some metabolic side effects of cefotaxime are transient lactate dehydrogenase elevations; however, the frequency of same has also been not reported.
- Cefotaxime can also cause joint discomfort as musculoskeletal side effects but same is reported in rare cases.
- Cefotaxime can also cause potentially fatal arrhythmias, shock, and hemorrhage as per post-marketing reports..
What to do if I overdose cefotaxime?
In neonatal, the acute toxicity was evaluated with the overdose of cefotaxime. In case of the dose in excess i.e. 6000 mg/kg/day parenterally, the significant mortality was seen in all groups.
The acute toxicity of Cefotaxime was evaluated in neonatal and adult mice and rats. Significant mortality was seen at parenteral doses in excess of 6000 mg/kg/day in all groups. Common toxic signs in animals that died were a decrease in spontaneous activity, tonic and clonic convulsions, dyspnea, hypothermia, and cyanosis. Cefotaxime sodium overdosage has occurred in patients.
Most cases have shown no overt toxicity. The most frequent reactions were elevations of BUN and creatinine. There is a risk of reversible encephalopathy in cases of administration of high doses of beta-lactam antibiotics including Cefotaxime. No specific antidote exists. Patients who receive an acute overdosage should be carefully observed and given supportive treatment.
Can cefotaxime interact with sodium intake?
Cefotaxime sodium contains approximately 51 mg (2.2 mEq) per each gram in parenteral form. The frozen solutions of cefotaxime sodium are additionally formulated with sodium hydrate as a buffer. In patients with conditions that may require sodium restriction (such as congestive heart failure, hypertension, and fluid retention), the sodium content should be considered.
Can cefotaxime be taken in patients with renal dysfunction?
Cefotaxime comes under the category of beta-lactam antibiotics, and these beta-lactam antibiotics are eliminated by the kidneys as unchanged drug or in its metabolites. In patients with impaired renal function, the serum concentrations of beta-lactam antibiotics and their metabolites may be increased and the half-lives can also be prolonged.
In patients who are treated with these agents parenterally, some effects have also been reported such as neurotoxic reactions including encephalopathy, asterixis, myoclonus, seizures, and coma.
If a patient has impaired renal function, the dose needs to be adjusted and also modifications should be based on the degree of renal impairment as well as the severity of infection in accordance with the individual product package labeling.
Nephrotoxicity can occur and also alterations in kidney function sometimes can occur with the use of these medications in high or prolonged usage, therefore, the renal functions test should be performed periodically.
Can cefotaxime be given in patients with colitis?
In patients who have been treated with antibacterial agents such as cefotaxime, pseudomembranous colitis have been reported, and the severity of colitis could range from mild to life-threatening, with an onset of up to two months, which can result in cessation of the therapy. The normal flora of colon can be altered by the antibiotic therapy and also it can permit the overgrowth of Clostridium difficle.
And the primary cause of antibiotic-associated colitis is known as caused by toxins of Clostridium difficile. The colitis is usually characterized by severe, persistent diarrhea and severe abdominal cramps, and may be associated with the passage of blood and mucus. Clindamycin, lincomycin, the aminopenicillins, and cephalosporins (cefotaxime) are considered as main culprits for colitis.
In patients who have a history of gastrointestinal diseases (especially colitis), the agents with antibacterial activity and therapy with broad-spectrum antibiotics should be used with caution. Pseudomonas colitis may be associated with flares of underlying disease and the condition may need a more severe course in such patients.
If diarrhea occurs during therapy with antibiotics, the offending antibiotics should be discontinued. Stool cultures for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically. In order to establish a definitive diagnosis of severe diarrhea, a large bowel endoscopy should be considered.
Can cefotaxime be given in patients who are on dialysis?
Hemodialysis can remove the cefotaxime, and other cephalosporins. Doses should be scheduled for administration of medication after dialysis. One can also give the supplemental doses after dialysis. However, there are some medications such as cefonicid, cefixime, and ceftriaxone are not significantly removed by hemodialysis.
Can cefotaxime be given in patients with liver disease?
The use of cefotaxime (or certain cephalosporins) can cause hepatitis. There has transient increase in SGOT, SGPT, and alkaline phosphatase levels have also been observed. In patients with hepatic disorders, such medications should be used with extensive caution and proper monitoring.
Can cefotaxime cause seizure disorders?
In patients with renal impairment, and especially when doses were not reduced, several cephalosporin triggered seizures. The drug should be discontinued immediately if seizures occur with these medications. If necessary or clinically indicated, an anticonvulsant therapy may be initiated. One should monitor patients with preexisting seizure disorders.
Can cefotaxime be given to a patient who received cholera vaccine recently?
You should always talk to your doctor before receiving cholera vaccine live if you are treated with cefotaxime recently or treated within the last 14 days. The activity of vaccine can be reduced by the cefotaxime.
You should not receive cholera vaccine until 14 days after you complete cefotaxime therapy, in order to ensure adequate vaccine response. If you are taking other medications such as vitamins and herbs, you should let your doctor know about this. You should not even stop taking any medications without prior consultation to the doctor.
Can cefotaxime be taken with warfarin?
You should not take cefotaxime along with warfarin because the anticoagulant effects of vitamin K antagonists can be potentiated by the cefotaxime or other cephalosporins. It can occur due to the inhibited growth of vitamin K producing intestinal bacteria, inhibited production of vitamin k dependent clotting factors via a methylthiotertrazole (MTT) side chain or may be due to inhibited platelet activity.
During treatment with cephalosporins or cefotaxime, the prothrombin activity can be reduced. Renal or hepatic impairment, poor nutritional state, antimicrobial therapy and chronic anticoagulation therapy are some risk factors associated with concurrent administration of warfarin and cefotaxime.
The patient should be monitor closely in terms of prothrombin time and signs of bleeding should also be watched. The anticoagulant dose may be adjusted as indicated. If patient experience any signs and symptoms of excessive anticoagulation, such as unusual or prolonged bleeding, bruising, coffee ground emesis, change in stool or urine color, headache, dizziness, or weakness, they should notify their doctors immediately.
Can cefotaxime be taken with furosemide?
Antibiotics like cephalosporins including cefotaxime can cause kidney problems occasionally; the risk of kidney problem can be increased due to concurrent administration of furosemide. The interaction between cefotaxime and furosemide get increased when the high doses are given directly into the veins and also when given in elderly patients with impaired kidney functions.
Nausea, vomiting, loss of appetite, increased or decreased urination, sudden weight gain or weight loss, fluid retention, swelling, shortness of breath, muscle cramps, tiredness, weakness, dizziness, confusion, and irregular heart rhythm are some symptoms of kidney damage that may occur with concurrent administration of both the medications.
During treatment, if you also suffer from any such signs or side effects, let your doctor know about it. Your doctor may adjust the dose or manage frequent monitoring for you or he may also use these medications with extra precaution in you. Other medications such as vitamins or herbs, if you are taking already, let your doctor know about this too. You should also not stop any medications without first talking to your doctor.
Can cefotaxime be taken with amikacin?
Again the risk of kidney damage is increased when cefotaxime is administered along with amikacin. If you have any concern, you should talk to your doctor. For prescribing you this combination, your doctor may monitor you closely for any potential complications and also after knowing all the risks that can happen, he will choose the best suitable dose of this medication for you, therefore it is necessary to always talk to your doctor.
Nausea, vomiting, loss of appetite, increased or decreased urination, sudden weight gain or weight loss, fluid retention, swelling, shortness of breath, muscle cramps, tiredness, weakness, dizziness, confusion, and irregular heart rhythm are some symptoms and signs of kidney damage that can occur with the concurrent administration of cefotaxime and furosemide.
Let your doctor know if you experience some or all of these problems during treatment. It is important to tell your doctor about all other medications you use, including vitamins and herbs. Do not stop using any medications without first talking to your doctor.
Can cefotaxime be given with chloramphenicol?
Chloramphenicol and cefotaxime can together cause moderate interaction. In the treatment of certain infections, chloramphenicol can diminish the effects of cefotaxime. You should talk to your doctor in case of any concerns regarding concurrent administration of cefotaxime and chloramphenicol.
Your doctor may be able to prescribe alternatives that do not interact, or you may need a dose adjustment or more frequent monitoring by your doctor to safely use both medications. It is important to tell your doctor about all other medications you use, including vitamins and herbs. Do not stop using any medications without first talking to your doctor.
Can cefotaxime be taken with ethyl estradiol?
Concurrent administration of cefotaxime and ethyl estradiol can cause moderate interaction. You may be at increased risk of pregnancy or breakthrough bleeding, if you are taking ethinyl estradiol for birth control. Your doctor may prescribe you another form of birth control during or after treatment, therefore you should talk to your doctor regarding this.
You can also ask if you can use some alternative birth control medication or method. You should tell your doctor about all other medications including vitamins and herbs and also you should not stop any medications without proper consent of the doctor.
Can cefotaxime be taken with typhoid vaccine?
You should talk to your doctor before receiving typhoid vaccine, if you are currently being treated with cefotaxime or have been treated within last 3 days. The activity of the vaccine can be reduced by antibiotics like cefotaxime. You should not receive typhoid vaccine live, until 3 days after you complete cefotaxime therapy in order to ensure adequate vaccine response.
You should wait for at least 3 days before using cefotaxime, if you have been vaccinated recently. If you need treatment for an infection, your doctor may be able to prescribe a different antibiotic. It is important to tell your doctor about all other medications you use, including vitamins and herbs. Do not stop using any medications without first talking to your doctor.
“What is the drug Dilantin used for? What are the side effects of too much Dilantin?“