Contents
- What is naltrexone?
- What are the indications and usage of naltrexone?
- How to administer naltrexone?
- How to treat alcoholism with naltrexone?
- How to treat opioid dependence with naltrexone?
- Can naltrexone be taken in pregnancy?
- Can naltrexone be given to nursing mothers?
- Is it safe to use naltrexone in pediatrics?
- What are the side effects of naltrexone?
- What are the contradictions of naltrexone?
- Can naltrexone be responsible for opioid overdose?
- Can naltrexone cause precipitate opioid withdrawal?
- Can naltrexone cause hepatotoxicity?
- Can naltrexone cause depression and suicidality?
- Can naltrexone be used for ultra-rapid opioid withdrawal?
- Can naltrexone be identified by laboratory tests?
- What are the drugs that can interact with naltrexone?
- What are reported adverse events of naltrexone?
- Can alcoholism be an adverse event of naltrexone?
- Can naltrexone promote opioid addiction?
- Is naltrexone cause drug dependence?
- What happens if I overdose naltrexone?
- How to treat naltrexone overdose?
- How to switch to naltrexone from other medications (Buprenorphine, naloxone or methadone)?
What is naltrexone?
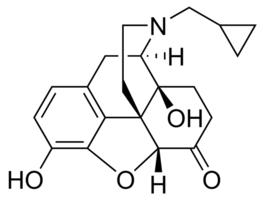
Naltrexone is a derivative of noroxymorphone which is the N-cyclopropylmethyl congener of naloxone. It is narcotic antagonist which is more potent than naloxone, long-lasting and more effective while taken orally. It is an FDA approved treatment for alcohol dependence.
Naltrexone is given to the patients who are dependent on opioids, for blocking the effects of opioid medications. Such effects include pain relief or euphoria feeling which can again be responsible for opioid abuse. Naltrexone is a part of treatment for drug alcohol dependence nowadays.
When people stop using opioid medications, they may suffer from severe withdrawal and relapse, naltrexone effectively used for preventing the relapse in opioid abusers. Naltrexone is considered as best medication when taken for treating opioid dependence because it stops the need of opioid in you.
Apart from treating the opioid dependence, naltrexone is also used for treating the alcohol dependence. This is the medication that can make you stop taking the alcohol completely; however, one should consider the treatment program under the supervision of doctor. Naltrexone can be benefitted in treating the alcohol dependence but is not helpful in reducing the effects of alcohol that you already consumed.
What are the indications and usage of naltrexone?
Naltrexone is not used to provide any therapeutic effects but used significantly as a part of addiction treatment plan. Naltrexone is indicated for the treatment of:
- Alcohol dependence
- Blockage of effects of opioids that administered exogenously
How to administer naltrexone?
Before starting naltrexone tablets treatment, one should make sure that the patients dependent on opioids, or exacerbation of a preexisting subclinical withdrawal syndrome, opioid dependent patients (including those being treated for alcohol dependence), should be opioid-free, in order to reduce the risk of precipitated withdrawal.
Naltrexone should be given after an opioid free interval of 7 to 10 days minimum as recommended for patients who were previously dependent on short acting opioids.
How to treat alcoholism with naltrexone?
In the treatment of alcoholism, a dose of 50 mg once daily is recommended for most alcoholic patients. A dosing regimen of naltrexone 50 mg twice daily for up to 12 weeks, demonstrated the efficacy of naltrexone as an adjunctive treatment of alcoholism. Other dose regimens or durations of therapy were not evaluated in these trials.
- A successful treatment of alcoholism is based upon many factors and naltrexone tablets should be considered as only one of many factors.
- The type of treatment, intensity, and duration of treatment; appropriate management of comorbid conditions; use of community-based support groups; and good medication compliance were the factors associated with a good outcome in the clinical trials with naltrexone.
- Appropriate compliance-enhancing techniques should be implemented to achieve the best possible treatment outcome. Such techniques should be the main components of the treatment program, especially medication patient compliance.
How to treat opioid dependence with naltrexone?
Treatment should be initiated with an initial dose of 25 mg of naltrexone tablets. The patient can be transferred to 50 mg a day if no withdrawal symptoms occur in patients.
For adequate clinical blockade of actions of parenterally administered opioids, a dose of 50 mg once a day should be administered. As with many non-agonist treatments for addiction, naltrexone tablets are of proven value only when given as part of a comprehensive plan of management that includes some measure to ensure the patient takes the medication.
Can naltrexone be taken in pregnancy?
Naltrexone comes under pregnancy category C, it shows an increase in the incidence of early fetal loss when given in doses>30mg/kg/day, which is 5 times of the recommended therapeutic dose.
These studies were done on the rats. However, the evidence of teratogenicity was not observed on oral doses. Major human metabolites, 6-β-naltrexol does not form in rats. Therefore, the potential reproductive toxicity of the metabolites in rats is not known.
There are no adequate and well-controlled studies have done in pregnant women. Naltrexone should be used during pregnancy with extreme cautions and should be used only if the potential benefit justifies the potential risk to the fetus. Effect of naltrexone on duration of labor and delivery is not known. Therefore it should not be used in such conditions, until and unless advised by your doctor.
Can naltrexone be given to nursing mothers?
In rats, naltrexone and its metabolite 6-β-naltrexol have been observed excreted in the milk, when given naltrexone orally.
However, the studies are not shown to be excreted in human milk and there are no evidence found. A nursing woman should take this medication with precautions, by keeping in mind the fact that there are many other medications that occurred in human milk.
Is it safe to use naltrexone in pediatrics?
The safe use of Naltrexone hydrochloride in pediatric patients younger than 18 years old has not been established.
What are the side effects of naltrexone?
Opioid withdrawal symptoms can be stimulated when you are taking naltrexone while using opioid medicine. One can suffer from withdrawal symptoms such as yawning, irritability, sweating, fever, chills, shaking, vomiting, diarrhea, watery eyes, runny nose, goose bumps, body aches, trouble sleeping, and feeling restless, when naltrexone and opioid administered concomitantly.
You should contact your healthcare provider if you have signs of allergic reactions such as hives, difficulty breathing, and swelling of face, lips, tongue, or throat. You should call your doctor if you see these side effects of naltrexone:
- Severe nausea, vomiting, or diarrhea
- Mood changes, confusion, hallucinations (seeing or hearing things)
- Depression, thoughts about suicide or hurting yourself
- Liver problems–nausea, upper stomach pain, itching, tired feeling, loss of appetite, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes)
Common side effects may include:
- Nausea, vomiting, stomach pain
- Headache, dizziness, drowsiness
- Feeling anxious or nervous
- Sleep problems (insomnia)
- Muscle or joint aches
What are the contradictions of naltrexone?
Naltrexone is contraindicated in:
- Patients receiving opioid analgesics.
- Opioids dependent patients which include the patients who are on opiate agonists for example methadone or partial agonists (such as Buprenorphine).
- Patients in acute opioid withdrawal
- Any individual who has failed the naloxone challenge test
- Patients who have positive urine screen for opioids.
- Any individual with a history of sensitivity to Naltrexone hydrochloride or any other ingredient of this product.

Can naltrexone be responsible for opioid overdose?
Shortly after completing detoxification, patients are likely to have reduced tolerance to opioids after opioid detoxification. Patients who have been treated with naltrexone may respond to lower doses of opioids than previously used due to the blockade of exogenous opioids provided by Naltrexone hydrochloride wanes and eventually dissipates completely.
This could result in potentially life-threatening opioid intoxication (respiratory compromise or arrest, circulatory collapse, etc.) if the patient uses previously tolerated doses of opioids. After discontinuing treatment, cases of opioid overdose with fatal outcomes have been reported in few patients.
After naltrexone treatment is discontinued, the patients should be alerted that they may be more sensitive to opioids, even at lower doses. Similarly family of the patients should be informed about the increased sensitivity to the opioids and the risk of overdose.
Patients also have the possibility to overcome the opioid blockade effect of naltrexone. The opioid blockade effect of naltrexone is surmountable, even after having the potent antagonist activity of naltrexone.
The plasma concentration of exogenous opioids attained immediately following their acute administration may be sufficient to overcome the competitive receptor blockade. In order to overcome the blockade by administering large amounts of exogenous opioids, patient can develop a potential risk.
It is extremely dangerous to overcome the antagonism by taking opioids by patients as it may lead to life-threatening opioid intoxication or fatal overdose. One should tol the patients and their family members or loved ones about the serious consequences of trying to overcome the opioid blockade.
Can naltrexone cause precipitate opioid withdrawal?
The withdrawal symptoms which are associated with the discontinuation of opioid in a dependent individual are very uncomfortable but they are not very severe or necessitate hospitalization.
However, the resulting withdrawal syndrome can be severe enough that require hospitalization when withdrawal is precipitated abruptly by the administration of an opioid antagonist to an opioid-dependent patient.
Within 5 minutes of ingestion of naltrexone, symptoms of withdrawal start appearing that lasted for 48 minutes. Mental status changes including confusion, somnolence and visual hallucinations have occurred.
Intravenous fluid administration may be required after significant fluid losses from vomiting and diarrhea. Symptoms of withdrawal severe enough to require hospital admission (and in some cases, management in the intensive care unit) have been identified with review of post-marketing cases of precipitated opioid withdrawal in association with naltrexone treatment.
In patients dependent on opioids, or exacerbation of a pre-existing subclinical withdrawal syndrome, opioid-dependent patients, including those being treated for alcohol dependence, should be opioid-free (including tramadol) in order to prevent occurrence of precipitated withdrawal, before starting naltrexone treatment.
In patients previously dependent on short acting opioids, an opioid-free interval of a minimum of 7 to 10 days is recommended. The precipitation of withdrawal symptoms that lasts for as long as two weeks, can be occur in patients transitioning from Buprenorphine or methadone.
Monitor the patient closely in an appropriate medical sitting in case a more rapid transition from agonist to antagonist therapy is deemed necessary and appropriate by the healthcare provider. The patient should be placed where precipitated withdrawal can be managed.
As there is no completely reliable method for determining whether a patient has an appropriate opioid free period, the healthcare providers should always prepared to manage the withdrawal with non-opioid medications symptomatically.
A naloxone challenge test may be helpful in determining the opioid free level of a patient; however, a few case reports have indicated that patients may experience precipitated withdrawal despite having a negative urine toxicology screen or tolerating a naloxone challenge test (usually in the setting of transitioning from buprenorphine treatment).
One should aware the patients about the risks associated with precipitated withdrawal and encouraged to give an accurate account of last opioid use. Patients treated for alcohol dependence with naltrexone should also be assessed for underlying opioid dependence and for any recent use of opioids prior to initiation of treatment with naltrexone.
Precipitated opioid withdrawal has been observed in alcohol-dependent patients in circumstances where the prescriber had been unaware of the additional use of opioids or co-dependence on opioids.
Can naltrexone cause hepatotoxicity?
During clinical development program and in post-marketing period, while taking naltrexone, few cases are reported which are:
- Hepatitis and clinically significant liver dysfunction
- Transient, asymptomatic hepatic transaminase elevations
There could be many other etiologies that can contribute to conditions like hepatitis, when patients are presented with elevated transaminase. Such etiologies may include pre-existing alcoholic liver disease, hepatitis B and/or C infection, and concomitant usage of other potentially hepatotoxic drugs.
Only the opioid withdrawal cannot be considered responsible for liver dysfunction. A systemic sequelae of actions can occur with opioid withdrawal which may include liver injury.
Patient should seek medical emergency in case he/she see any signs of liver insufficiency or hepatic injury or if they see any symptoms of acute hepatitis. The naltrexone should be discontinued immediately in the symptoms of hepatitis, no matter if it responsible for hepatitis or not.
Can naltrexone cause depression and suicidality?
In the post-marketing experience of naltrexone, in patients with opioid dependence, depression, attempted suicide, and suicidal ideation have been reported. However, there is no casual relationship has been demonstrated. The patients should be monitored carefully for the development of depression or suicidal thinking, if they are opioid dependent and taking naltrexone.
The emergences of symptoms of depression or suicidality are common in patients who are taking naltrexone therefore the family members of the patients should be alerted to observe such symptoms and also report them to the doctor at earliest.
Can naltrexone be used for ultra-rapid opioid withdrawal?
Safe use of Naltrexone in ultra rapid opiate detoxification programs has not been established.
Can naltrexone be identified by laboratory tests?
Naltrexone hydrochloride does not interfere with thin-layer, gas-liquid, and high pressure liquid chromatographic methods. These are the methods which may be used for the separation and detection of morphine, methadone or quinine in the urine.
For detection of opioids depending on the specificity of the test, naltrexone may or may not interfere with enzymatic methods. You should consult with your doctor regarding specific details of the test for naltrexone.
What are the drugs that can interact with naltrexone?
There are not many studies that are conducted in order to check the interaction of naltrexone with other drugs; however, you should take this medication with extreme caution or only after proper consultation with doctor, if you are taking with any other medication.
Naltrexone and disulfiram: The safety of concurrent administration of both drugs is unknown, but both the medications are known to have potential for hepatotoxicity. Therefore use of these medications altogether should be avoided unless the benefit outweighs the risk.
Naltrexone and thiordazine: When both these medications are take concomitantly, can cause lethargy and somnolence.
Patients taking Naltrexone hydrochloride may not benefit from opioid containing medicines, such as cough and cold preparations, ant diarrheal preparations, and opioid analgesics.
The amount of opioid required may be greater than usual in an emergency situation when opioid analgesia must be administered to a patient receiving naltrexone. This can result in a deeper and prolonged respiratory depression.
What are reported adverse events of naltrexone?
In patients who are known to be free of opioids for more than 7 to 10 days, naltrexone has not been seen to cause no increase in complaints significantly. Small fraction of patients may experience from opioid withdrawal symptom complex consisting of tearfulness, mild nausea, abdominal cramps, restlessness, bone or joint pain, myalgia, and nasal symptoms, as per studies in alcoholic populations and volunteers.
This may represent the unmasking of occult opioid use, or it may represent symptoms attributable to naltrexone. In order to try to reduce the frequency of these complaints, a number of alternative dosing pattern have been recommended.
There is no identification of any single, serious untoward risk of naltrexone use in detoxified, formerly opioid-dependent individuals as per extensive clinical studies.
In placebo-controlled studies employing up to five-fold higher doses of Naltrexone hydrochloride (up to 300 mg per day) than that recommended for use in opiate receptor blockade have shown that Naltrexone hydrochloride causes hepatocellular injury in a substantial proportion of patients exposed at higher doses.
Aside from this finding, and the risk of precipitated opioid withdrawal, available evidence does not incriminate naltrexone used at any dose, as a cause of any other serious adverse reaction for the patient free from opioid addiction. It is critical to recognize that naltrexone can precipitate or exacerbate abstinence signs and symptoms in any individual who is not completely free of exogenous opioids.
Can alcoholism be an adverse event of naltrexone?
In a very few patients (2% in 570 patients) with alcoholism receiving naltrexone shows adverse events such as nausea (10%), headache (7%), dizziness (4%), nervousness (4%), fatigue (4%), insomnia (3%), vomiting (3%), anxiety (2%) and somnolence (2%). When comparing naltrexone, placebo or controls undergoing treatment for alcoholism, depression, suicidal ideation, and suicidal attempts have been reported in all groups.
Although no causal relationship with naltrexone is suspected, physicians should be aware that treatment with naltrexone does not reduce the risk of suicide in these patients.
Can naltrexone promote opioid addiction?
During naltrexone trials, in opioid addiction at a rate of more than 10%, the adverse reactions have been reported are difficulty sleeping, anxiety, nervousness, abdominal pain/cramps, nausea and/or vomiting, low energy, joint and muscle pain, and headache.
The incidence was less than 10% for adverse events like loss of appetite, diarrhea, constipation, increased thirst, increased energy, and feeling down, irritability, dizziness, skin rash, delayed ejaculation, decreased potency, and chills.
A very few and rare adverse events that occurred in <1% of patients are:
- Respiratory:
Nasal congestion, itching, rhinorrhea, sneezing, sore throat, excess mucus or phlegm, sinus trouble, heavy breathing, hoarseness, cough, shortness of breath.
- Cardiovascular
Nose bleeds, phlebitis, edema, increased blood pressure, non-specific ECG changes, palpitations, tachycardia.
- Gastrointestinal: Excessive gas, hemorrhoids, diarrhea, ulcer.
- Musculoskeletal: Painful shoulders, legs or knees; tremors, twitching.
- Genitourinary: Increased frequency of, or discomfort during, urination; increased or decreased sexual interest.
- Dermatologic: Oily skin, pruritus, acne, athlete’s foot, cold sores, alopecia.
- Psychiatric: Depression, paranoia, fatigue, restlessness, confusion, disorientation, hallucinations, nightmares, bad dreams.
- Special senses: Eyes blurred, burning, light sensitive, swollen, aching, strained; ears–”clogged,” aching, tinnitus.
- General: Increased appetite, weight loss, weight gain, yawning, somnolence, fever, dry mouth, head “pounding,” inguinal pain, swollen glands, “side” pains, cold feet, “hot spells.”
Is naltrexone cause drug dependence?
Naltrexone does not lead to physical dependence as it is purely an opioid antagonist. There are no reports show the occurrence of tolerance to opioid antagonist.
What happens if I overdose naltrexone?
There is limited clinical experience with Naltrexone hydrochloride over dosage in humans. There were no signs of toxicity were seen in a patients having higher doses (i.e. 800 mg) daily for one week, as per one study.
However, studies shows that as per animal studies, naltrexone at higher doses produced salivation, tremors, and convulsions and also mortalities were seen in animals due to convulsions mainly clonic-tonic type and also respiratory failure.
How to treat naltrexone overdose?
Patients, who overdosed naltrexone, should be treated symptomatically in a closely supervised environment. The overdose of naltrexone can be occur in lack of actual experience. A poison control center should be contacted for up to date information.
How to switch to naltrexone from other medications (Buprenorphine, naloxone or methadone)?
There is no specific and systemic data is given that proves the switching from buprenorphine or methadone to Naltrexone hydrochloride tablets is feasible; however, patients may suffer from manifestations of precipitated withdrawal when switched from opioid agonist to pure opioid antagonist therapy.
Patients transitioning from buprenorphine or methadone may be vulnerable to precipitation of withdrawal symptoms for as long as 2 weeks. Healthcare providers should be prepared to manage withdrawal symptomatically with non-opioid medications.
“What is the drug G made of? How long does the drug G stay in your system?“