Contents
- What is Prolintane?
- Prolintane IUPAC name, chemical structure, mass, formula
- Prolintane uses
- Prolintane brand names
- Prolintane legal status
- Prolintane effects
- Prolintane mechanism of action
- Prolintane pharmacokinetics
- Prolintane side effects
- Prolintane testing
- Can prolintane be detected on standard drug tests?
- Prolintane abuse and addiction
- Prolintane withdrawal
- Prolintane lethal dose
What is Prolintane?
Prolintane is a generic name for sympathomimetic amine drug with that works similarly as d-amphetamine. It has been marketed in Europe since the 1960s as an antidepressant (i.e., antifatigue), analeptic, and vasopressor (i.e., orthostatic hypotension) agent. The first reports of prolintane abuse appeared in Europe during the early 2000s when prolintane was identified in tablets distributed at a rave party (i.e., all- night dancing with electronically synthesized music). Later reports documented the recreational use of prolintane in the United States.
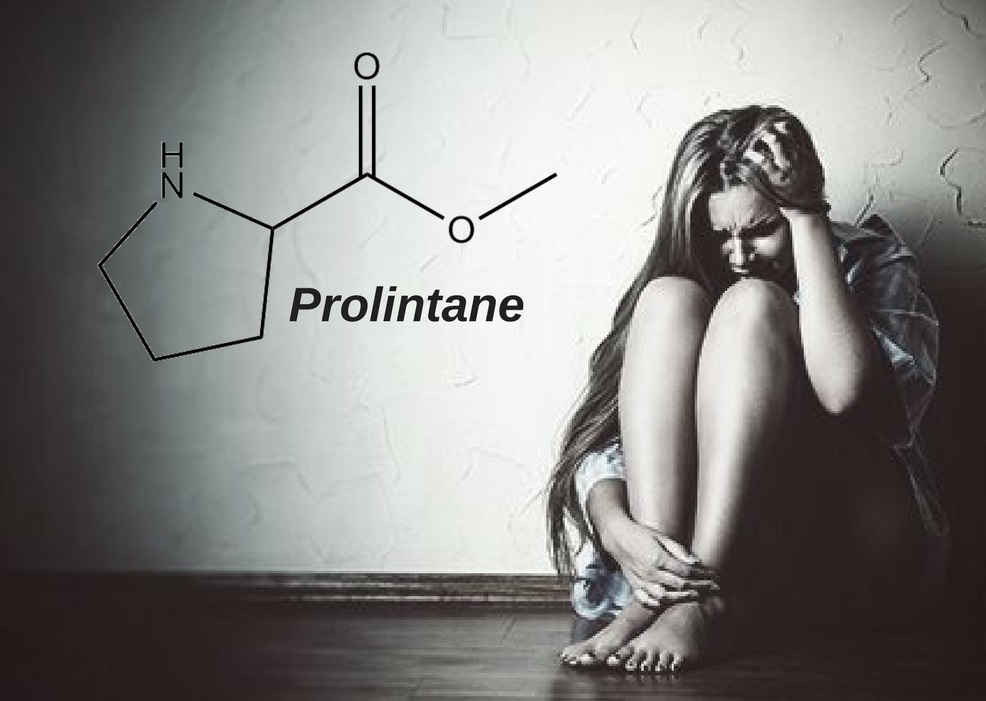
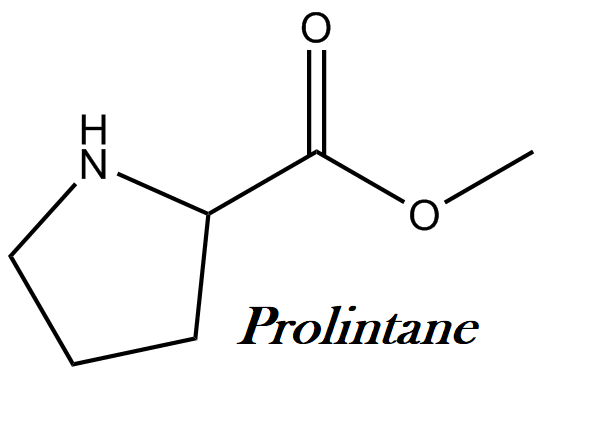
Prolintane IUPAC name, chemical structure, mass, formula
IUPAC name: 1-(1-phenylpentan-2-yl)pyrrolidine
Molecular formula: C15H23N
Molecular weight: 217.35 g/mol
Molecular structure:
Prolintane uses
Therapeutic uses of prolintane in Africa, Europe, and Australia include the treatment of narcolepsy, attention deficit hyperactivity disorder (ADHD), fatigue, and orthostatic hypotension. Prolintane is formulated with multivitamins including ascorbic acid and sold as an orange tablet (e.g., “ kitovit ” ) or a tonic.
Prolintane brand names
Proprietary preparations include Villescon ® (Boehringer Ingelheim, Berkshire, UK), Promotil ® (Boehringer Ingelheim, Paris, France), and Catovit ® (Boehringer Ingelheim, New South Wales, Australia).
Prolintane legal status
Prolintane is not approved for pharmaceutic use in the United States. Additionally, prolintane has been implicated as a doping agent in athletics (e.g., cycling); this compound is now banned by the US National Collegiate Athletic Association and the World Anti – Doping Agency (WADA).
Prolintane effects
Typical adult therapeutic doses of prolintane are 10 – 40 mg daily. In therapeutic trials, 20 mg prolintane is a mild stimulant equivalent to approximately 100 mg caffeine. The use of therapeutic doses of prolintane is associated with a subjective feeling of improved concentration and decreased fatigue, but there are minimal changes in objective measures of fatigue and mental activity.
The stimulant properties of prolintane are substantially less than d-amphetamine. In a study of fatigued volunteers, the administration of 20 mg or 40 mg prolintane produced similar, but less intense effects than 20 mg d-amphetamine. These clinical effects included stimulation, euphoria, anorexia, and mild elevation of systolic blood pressure.
In experimental studies of healthy volunteers, prolintane has little cardiovascular activity following the administration of a single dose of 20 mg. This dose slightly improves some mental tasks (e.g., sign recordings), but not others (e.g., digit span).
The clinical significance of these results in memory, learning, and concentration is unclear. In volunteer studies, the administration of prolintane (20 – 40 mg) increases wakefulness and improves performance on some perceptual and arithmetic tasks in fatigued individuals.
Prolintane mechanism of action
Prolintane is a CNS stimulant with many pharmacologic properties similar to d – amphetamine. However, rodent studies indicate that prolintane enhances brain dopamine turnover produced by dopamine receptor antagonists similar to methylphenidate and cocaine, whereas amphetamine and methamphetamine do not cause similar effects.
Prolintane pharmacokinetics
The first – pass effect of prolintane is large. Prolintane undergoes extensive oxidation and N – dealkylation in the body with the formation of at least 18 metabolites. The metabolism of prolintane primarily involves biotransformation.
The major metabolic pathways are aromatic hydroxylation ( para – and meta – positions), lactam formation, γ – amino acid formation, heterocyclic hydroxylation (3 – and 4 – positions), and propyl hydroxylation at ω-, ( ω – 1) – positions. Oxidation of the α – carbon of the pyrrolidine ring forms the major metabolite, oxoprolintane.
In a study of 5 healthy volunteers receiving 0.15 mg prolintane/kg intravenously, the mean plasma elimination half – life was approximately 4.5 hours. The urine pH of these volunteers was within the normal range. The kidney excretes minimal amounts (i.e., < 1 – 2%) of prolintane unchanged.
Prolintane side effects
Adverse effects of therapeutic doses include nausea, vomiting, anxiety, irritability, insomnia, lightheadedness, headache, euphoria, palpitations, diaphoresis, confusion, agitation, disorientation, and acute psychosis. Clinical effects associated with the abuse of prolintane include lightheadedness, insomnia, irritability, and nervousness.
Excessive use of prolintane may also cause hypertension, tachycardia, hyperrefl exia, mydriasis, hyperthermia, agitation, confusion, disorientation, hallucinations, and psychosis.
Prolintane testing
Analytic methods for the detection of prolintane include thin – layer chromatography, gas chromatography with nitrogen – selective detection, high performance liquid chromatography with ultraviolet detection (252 nm, 258 nm, 264 nm), gas chromatography/mass spectrometry, and capillary zone electrophoresis with β – cyclodextrin – modified micellar electrokinetic chromatography.
The limit of detection (LOD) and lower limit of quantitation (LLOQ) for the latter method were 300 ± 100 ng/mL and 1,000 ± 100 ng/mL, respectively. Prolintane is 1 of 14 tertiary amine stimulants detected by screening and confirmation with gas chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry.
The LOD in urine samples using this method is 10 ng/mL; this method meets WADA ’ s requirements for sensitivity. Following the intravenous (IV) injection of 0.15 mg prolintane/kg to 5 healthy volunteers, the mean peak plasma prolintane concentration was approximately 60 ng/mL.
Can prolintane be detected on standard drug tests?
Prolintane use is not usually detectable by routine urine immunoassay drug screens.
Prolintane abuse and addiction
The first reports of prolintane abuse was reported in Europe during the early 2000s when prolintane was identified in tablets distributed at a rave party. Later reports documented the recreational use of prolintane in the United States, where it appears to substitute for recreational amphetamine and other stimulant use.
Different from some of the other more potent stimulants in its class, the chronic use of prolintane can be considered mildly to moderately addictive with only a moderate potential for abuse and is capable of causing psychological dependence among certain users.
Prolintane withdrawal
When addiction has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage. Addiction is a potential risk among users of prolintane as it can cause compulsive redosing, although typically to a far lesser degree than the notoriously compulsive cathinone analogs A-PVP and MDPV.
Prolintane lethal dose
The precise lethal dose of prolintane remains unknown, and no formal studies have been carried out in humans. The typical adult therapeutic dose of prolintane has been 10-40mg daily. In therapeutic trials, 20mg prolintane is a mild stimulant equivalent to 100mg caffeine.
In a study of fatigued volunteers the administration of 20mg or 40mg prolintane produced similar, but less intense effects than 20mg d-amphetamine. In experimental studies of healthy volunteers, prolintane has little cardiovascular activity following the single dose of 20mg.
“Can you get addicted to lean the first time? Can lean kill you?“