Contents
- What is Saphris?
- Saphris molecular formula, weight, structure and drug class
- What are the indications and usage of Saphris?
- What is the pharmacology of Saphris?
- What is the pharmacokinetics of Saphris?
- How long Saphris stays in your system?
- What are the dosage forms and strengths of Saphris?
- What are the side effects of Saphris?
- How much does Saphris cost without insurance?
- Can Saphris be taken in pregnancy?
- Can Saphris be given to a nursing mother?
- Can Saphris be given to a patient with severe hepatic impairment?
- Can Saphris be given to a patient with dementia?
- Can Saphris be given to a patient with neuroleptics malignant syndrome (NMS)?
- Saphris and tardive dyskinesia?
- Can Saphris cause depression?
- Can Saphris be taken with alcohol?
- Can Saphris cause hyperglycemia?
- Is Saphris associated with high cholesterol levels?
- Do Saphris cause obesity?
- Can Saphris be taken with dolasetron?
- Can Saphris be taken with amiodarone?
- Can Saphris be taken with droperidol?
- Can Saphris be taken with fentanyl?
- Can Saphris be taken with tramadol?
What is Saphris?
Saphris is a medication that contains asenapine maleate, which is an antipsychotic agent and is available as a sublingual tablet. It usually comes in black cherry flavor. It is known to act by changing the chemicals in the brain. It is mainly used for the treatment of schizophrenia in adults and bipolar disorders in adults (>10 years old).

Saphris tablet contains 2.5 mg, 5 mg, or 10 mg asenapine. It may also contain other inactive ingredients such as gelatin, mannitol, sucralose, and black cherry flavor.
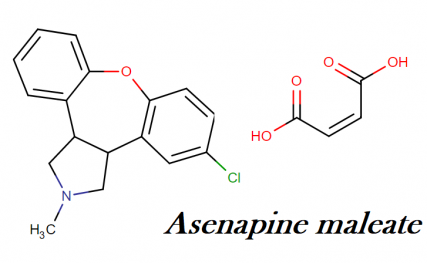
Saphris molecular formula, weight, structure and drug class
Molecular formula: C17H16CINO
Molecular weight: 285.771 g/mol
Molecular structure:
What are the indications and usage of Saphris?
Saphris is indicated for the treatment of mental disorders like schizophrenia and bipolar disorder. Saphris contains asenapine which acts on your thinking and making it clearer, you feel less nervous, even you start taking part in daily activities.
This medication also helps you in reducing the hallucinations and prevents mood swings. Saphris comes under the drug class atypical antipsychotics. It acts by restoring the balance of certain neurotransmitters in the brain. It is mainly indicated in:
- Schizophrenia
- Bipolar disorders: manic or mixed episodes associated with bipolar disorders
- Adjunctive therapy with lithium or valproate indicated for the treatment of bipolar disorders (manic or mixed episodes)

What is the pharmacology of Saphris?
Asenapine is a serotonin, dopamine, noradrenaline and histamine antagonist. This medication possesses more potent activity with serotonin receptors than dopamine. Antagonist activity of Saphris towards histamine receptors is responsible for its sedative properties. Its lower incidence of extrapyramidal effects is associated with the upregulation of D1 receptors.
This up-regulation occurs due to Saphris’s dose-dependent effects on glutamate transmission in the brain. Asenapine does not have any significant activity with muscurainic and cholinergic receptors, therefore, symptoms like dry mouth, constipation (associated with anticholinergic drug) are not expected to be observed.
What is the pharmacokinetics of Saphris?
- Bioavailability and absorption: Saphris have low bioavailability (<2%) and therefore administered sublingually, it passes via first pass metabolism which observed oral administration of the tablets. The absolute bioavailability of sublingual tablets (5mg) is 35%.
- It is rapidly absorbed in the sublingual, supralingual, and buccal mucosa following sublingual administration; peak plasma concentrations occur within 0.5–1.5 hours. Saphris reaches its steady-state plasma concentrations reached within 3 days of twice-daily administration.
- Immediately before food or having food 4 hours after taking this tablet (5 mg) sublingually; can reduce the exposure by 20 or 10% respectively, mainly due to increased hepatic flow.
- Water intake 2 and 5 minutes following sublingual administration of asenapine 10 mg in adults decreased exposure by 19 and 10%, respectively; effects of water intake 10 or 30 minutes after administration were equivalent.
- Distribution: Saphris is rapidly distributed and extensive extravascular distribution is indicated by the large volume of distribution.
- Saphris has 95% plasma protein binding including albumin and α1-acid glycoprotein.
- It is metabolized by direct glucuronidation and oxidative metabolism. It gets metabolized into primarily asenapine N-glucuronide, also N-desmethylasenapine and N-desmethylasenapine N-carbamoyl glucuronide which are largely inactive.
- About 90% of the dose was recovered; approximately 50% recovered in urine and 40% in feces, after administration of the single radiolabelled dose.
How long Saphris stays in your system?
Saphris has a half-life of 24 hours i.e. 1 day, and therefore it stays in your system for 5 days. And it will take almost 5 days to take this medication out of your system.
What are the dosage forms and strengths of Saphris?
- Saphris 5 mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with a hexagon on one side.
- Saphris 5 mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with “5” on one side within a circle.
- Saphris 10 mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with “10” on one side within a circle.
What are the side effects of Saphris?
Saphris (asenapine) is an atypical antipsychotic psychiatric medication used to treat certain mental/mood disorders (such as schizophrenia, bipolar disorder). Common side effects of Saphris include:
- Drowsiness
- Dizziness
- Numbness or tingling of the mouth
- Restlessness
- Constipation
- Dry mouth
- Insomnia
- Weight gain
- Upset stomach

Saphris may cause muscle/nervous system problems (extrapyramidal symptoms-EPS). Tell your doctor if you notice the following side effects of Saphris including:
- Feelings of anxiety/agitation/jitteriness
- Drooling or trouble swallowing
- Constant need to move
- Tremors
- Stiff muscles
- Severe muscle spasms or cramping such as twisting neck, arching back, eyes rolling up
- Mask-like expression of the face
How much does Saphris cost without insurance?
| Saphris Subl Dosage | Estimated Cost per unit (Each tablet) |
| 2.5 mg | $24.10 |
| 5 mg | $24.10 |
| 10 mg | $24.10 |
Can Saphris be taken in pregnancy?
Saphris comes under the USFDA pregnancy category – C. Drugs which owing to their pharmacological effects, have caused or may be suspected of causing, harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible.
The use of Saphris is not recommended in pregnancy until the benefits outweigh the risk. Neonates exposed to this drug during the third trimester should be monitored for extrapyramidal and/or withdrawal symptoms following delivery.
When given Saphris to the animal, there was no teratogenicity were observed however developmental toxicity was seen. In neonates exposed to antipsychotic during the third trimester, the symptoms like agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported.
This drug seems to elevate prolactin levels with other drugs that antagonize dopamine D2 receptors. Still, there are no controlled data in human pregnancy, therefore, one should avoid using this medication in pregnancy and should be taken only if benefits outweigh the risks.
Can Saphris be given to a nursing mother?
This medication is known to excrete in the human milk and therefore it should not be used by a nursing mother. As this medication excreted in human milk, there are no effects are known in a nursing infant.
Can Saphris be given to a patient with severe hepatic impairment?
Saphris is contraindicated in patients with severe hepatic impairment. The exposure of asenapine is 7 fold higher in patients with severe hepatic impairment as compared to normal patients with normal hepatic function as it is a high clearance drug. In patients with mild to moderate hepatic impairment, there is no dosage adjustment is required as the drug exposure is similar to that in subjects with normal hepatic function.
Can Saphris be given to a patient with dementia?
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death, mostly from cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) causes. A causal relationship with antipsychotic use has not been established. In controlled trials, treatment with some atypical antipsychotic drugs was also associated with an increased risk of cerebrovascular adverse events (e.g., stroke, transient ischemic attack), including fatalities, in elderly patients with dementia-related psychosis. These agents are not approved for the treatment of patients with dementia-related psychosis.
Can Saphris be given to a patient with neuroleptics malignant syndrome (NMS)?
Neuroleptic agents such as Saphris cause central dopaminergic blocking effects which can aggravate a potentially fatal symptom complex known as a neuroleptic malignant syndrome (NMS). When high potency agents like haloperidol are given intramuscularly or any neuroleptic agent gave for any length of time, the neuroleptic malignant syndrome can observe more frequently.
Clinical manifestations of NMS include hyperpyrexia, muscle rigidity, altered mental status and autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac arrhythmias). Elevated creatine phosphokinase, myoglobinuria, and acute renal failure are some additional signs.
In patients with active NMS, neuroleptics such as Saphris should not be given. One should immediately stop this medication if already giving to a patient with NMS. In patients with a history of NMS, introduction or reintroduction of neuroleptic agents should be carefully considered, since NMS may recur.
Saphris and tardive dyskinesia?
The symptoms of tardive dyskinesia can be precipitated by neuroleptic agents. Tardive dyskinesia is a syndrome syndrome consisting of rhythmic involuntary movements variously involving the tongue, face, mouth, lips, jaw, and/or trunk and extremities, following chronic use of at least several months but often years. Elderly patients, particularly women, are most susceptible.
Both the risk of developing the syndrome and the likelihood that it will become irreversible increase with the duration and total cumulative dose of neuroleptic therapy administered. However, patients may infrequently develop symptoms after relatively brief treatment periods at low dosages.
If tardive dyskinesia occurs during neuroleptic therapy, prompt withdrawal of the offending agent or at least a lowering of the dosage should be considered. Tardive dyskinesia symptoms may become more severe after drug discontinuation or a dosage reduction, but may gradually improve over months to years.
In patients with preexisting drug-induced tardive dyskinesia, initiating or increasing the dosage of neuroleptic therapy may temporarily mask the symptoms of tardive dyskinesia but could eventually worsen the condition.
The newer, atypical neuroleptic agents (e.g., risperidone, quetiapine, olanzapine) tend to be associated with a substantially reduced risk of inducing tardive dyskinesia and are considered the drugs of choice in patients being treated for psychosis.
Can Saphris cause depression?
While taking this medication, adult and pediatric patients with depression and other psychiatric disorders may experience worsening of their symptoms. They may also have the emergence of suicidal thoughts and behavior.
The worsening of the symptoms, suicidality or changes in one’s behaviors should be closely monitored. One should discontinue the medication if the symptoms persist or persistently getting worse or abrupt in onset.
Can Saphris be taken with alcohol?
The side effects of the Saphris such as dizziness, drowsiness, and difficulty in concentration can be increased with alcohol administration along with this medication. You can also suffer from the impaired thinking and poor judgment.
While treating with Saphris you should limit the use of alcohol. One should avoid the activities that require mental alertness such as driving or operating a heavy machinery which needs extra alertness and should also not use more than the recommended doses of Saphris. If you have any concern or confusion regarding this medication, you should always talk to your pharmacist or your doctor.
Can Saphris cause hyperglycemia?
The use of atypical antipsychotic agents has been reported to cause hyperglycemia which was associated with ketoacidosis or hyperosmolar coma or death. When treated with these agents, the patients should be monitored for worsening control of blood glucose.
At the beginning of the treatment, the patient with risk factors for diabetes mellitus should undergo fasting blood glucose testing, when taking atypical antipsychotic medications concurrently. The testing should also be done periodically thereafter. For symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness, patients (who are treated with atypical antipsychotic medications) should be monitored.
In some cases, hyperglycemia has resolved when treatment with these agents was discontinued; however, despite discontinuation of the atypical antipsychotic drug, some patients required continuation of anti-diabetic treatment.
Is Saphris associated with high cholesterol levels?
Undesirable lipid alterations in lipid levels can occur with atypical antipsychotic drugs. While all agents in the class have been shown to produce some changes, each drug has its own specific risk profile. Before or soon after initiation of antipsychotic medication, obtain a fasting lipid profile at baseline and monitor periodically during treatment.
Do Saphris cause obesity?
Typical antipsychotic medication use is usually associated with weight gain. Each drug from the antipsychotic class has its own specific risk profile along with many other changes. Weight gain should be monitored in pediatric patients, and one should also monitor the normal growth of the child. One should monitor weight at baseline and frequently thereafter.
Can Saphris be taken with dolasetron?
Dolasetron can turn into its active metabolite; hydrodolasetron which can cause dose-related prolongation of the QT interval and the additive effects can be caused by the concurrent administration of the dolasetron. The addictive effects can result in an increase the risk of ventricular arrhythmias including torsade de pointes and sudden death.
One should practice extreme caution if dolasetron is used in combination with cumulative anthracycline therapy (in high doses) or other agents that can increase the prolongation of QT interval. In high-risk patients, the monitoring should be appropriate.
Symptoms such as lightheadedness, fainting, palpitation, irregular heart rhythm, shortness of breath, dizziness or syncope should be considered immediately and patients should seek immediate medical attention.
Can Saphris be taken with amiodarone?
Amiodarone is a class III antiarrhythmic agent, other agents of this group are dofetilide and sotalol and they can cause dose-related prolongation of QT interval. One should generally avoid the concurrent administration of the agents that can cause prolonged QT interval along with the agents like agents like Saphris.
Because the co-administration of this agent can cause additive effects which can result in increased risk of ventricular arrhythmias including torsade de pointes and sudden death.
In general, the risk of an individual agent or a combination of agents causing ventricular arrhythmia in association with QT prolongation is largely unpredictable but may be increased by certain underlying risk factors such as congenital long QT syndrome, cardiac disease, and electrolyte disturbances (e.g., hypokalemia, hypomagnesemia). In addition, the extent of drug-induced QT prolongation is dependent on the particular drug(s) involved and dosages of the drugs.
Concurrent administration of class I and Class III antiarrhythmic agents with the drugs that are known to increase the QT interval should be generally avoided until the benefits outweigh the risks.
If concomitant use is necessary, the close monitoring of the patient is advised. Symptoms such as lightheadedness, fainting, palpitation, irregular heart rhythm, shortness of breath, dizziness or syncope should be considered immediately and patients should seek immediate medical attention.
Can Saphris be taken with droperidol?
The concurrent administration of asenapine with droperidol can cause major interaction. One should closely monitor the patient if these medications are given together. The monitoring is advised because the use of droperidol has been associated with QT interval prolongation, torsade de pointes and other serious arrhythmias, and sudden death.
The risk of prolonged QT syndrome can be increased by the concurrent of agents that are able to produce hypokalemia and/or hypomagnesemia (such as potassium-wasting diuretics, amphotericin B, cation exchange resins), and the agent s that can increase the QT interval (such as phenothiazines, tricyclic antidepressants, antiarrhythmic agents, etc.), certain other drugs (benzodiazepines, volatile anesthetics, intravenous opiates), or alcohol abuse.
In patients (mainly in elderly or debilitated patients) taking droperidol along with CNS depressants or respiratory depressants agents, the depressant action can be increased due to synergistic action.
In case it becomes necessary to give both the medications together, extreme caution and monitoring of the patient is advised. One should individualize and titrate the dose of droperidol, in order to achieve the desired effects. One should monitor the routing vital sign and ECG monitoring. The patient should be monitored for excessive or prolonged CNS or respiratory depression when the combination of droperidol and Saphris is given.
The patient should also know that how these agents can affect them and ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination. One should immediately notify the doctor if they experience any unusual activity like prolonged CNS effects that can interfere with their daily routine activities.
Can Saphris be taken with fentanyl?
One should generally avoid the concurrent administration of the opioids with benzodiazepines or other central nervous system depressants. The agents such as nonbenzodiazepine sedatives/hypnotics, anxiolytics, muscle relaxants, general anesthesia. Along with CNS depressants, ethics, antipsychotics, other opioids, alcohol are known as CNS depressants which can cause extreme sedation, respiratory depression, coma, and death.
Some CNS depressants can also enhance the risk of hypotension, for example, alcohol, benzodiazepines, phenothiazines. One should avoid the administration of opioids with benzodiazepines or CNS depressant until the unless the other treatment options are inadequate.
If it becomes necessary to coadminister both these medications, one should limit the dosage of these medications to the minimum in order to achieve the desired effect. If these medications are given together, the patient should be monitored closely for the symptoms of sedation and one should also advise to no driving and no heavy machinery work after taking this combination.
A doctor should not prescribe a cough preparation that contains codeine or hydrocodone along with benzodiazepines or other CNS depressants including alcohol. A patient should discontinue one of the medications of concurrently administering this combination, and the cessation of any drug should not be done abruptly whereas it should be stopped gradually.
The abrupt withdrawal of can causes severe withdrawal symptoms. The patient can suffer from numbness and tingling of the extremities, hypersensitivity to light and noise, hallucinations, and epileptic seizures as a result of withdrawal symptoms of benzodiazepines.
Can Saphris be taken with tramadol?
The concomitant use of Saphris and benzodiazepines or other central nervous system depressants should be avoided. Because the CNS depressants like nonbenzodiazepines sedative, hypnotics, anxiolytics, muscle relaxants, general anesthetics, antipsychotics, and alcohol can cause extreme sedation, respiratory depression, coma and even death in some cases.
Some CNS depressants can also enhance the risk of hypotension, for example, alcohol, benzodiazepines, phenothiazines. One should avoid the administration of opioids with benzodiazepines or CNS depressant until the unless the other treatment options are inadequate.
If it becomes necessary to coadminister both these medications, one should limit the dosage of these medications to the minimum in order to achieve the desired effect. If these medications are given together, the patient should be monitored closely for the symptoms of sedation and one should also advise to no driving and no heavy machinery work after taking this combination.
A doctor should not prescribe a cough preparation that contains codeine or hydrocodone along with benzodiazepines or other CNS depressants including alcohol. A patient should discontinue one of the medications of concurrently administering this combination, and the cessation of any drug should not be done abruptly whereas it should be stopped gradually.
The abrupt withdrawal of can causes severe withdrawal symptoms. The patient can suffer from numbness and tingling of the extremities, hypersensitivity to light and noise, hallucinations, and epileptic seizures as a result of withdrawal symptoms of benzodiazepines.
“What does Aripiprazole (Abilify) do to the brain? How long does it take for abilify to work for schizophrenia?”
“What is Cloderm cream used to treat?“