Contents
- What is adalimumab?
- Adalimumab molecular formula, weight and structure
- What is the mechanism of action of adalimumab?
- What are the indications of adalimumab?
- What are the contraindications of adalimumab?
- What are the warnings and precautions associated with the use of adalimumab?
- Pharmacokinetics of adalimumab
- How is adalimumab stored?
- Side effects of adalimumab
- Adalimumab dosage
- Adalimumab off-label use
- Adalimumab price and prescription
- Can I take adalimumab if I am pregnant?
- Can I take adalimumab if I am breastfeeding?
- Is adalimumab safe for children?
- Is adalimumab safe for elderly?
- Does adalimumab cause infertility?
- Can adalimumab cause cancer?
- What are some drugs that can interact with adalimumab?
- How long does adalimumab stay in your system?
What is adalimumab?
Adalimumab is a human monoclonal antibody that works against TNF-alpha, a pro-inflammatory molecule. Therefore it is used to treat chronic inflammatory conditions such as rheumatoid arthritis, Crohn’s disease etc. It is produced by recombinant DNA technology.
Since it is a protein, it cannot be given orally because it will be denatured in the stomach. So it is only given as an injection.
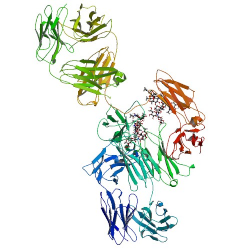
Adalimumab molecular formula, weight and structure
Molecular formula: C6428H9912N1694O1987S46
Molecular weight: 144190.3 Da
Molecular structure:
What is the mechanism of action of adalimumab?
Adalimumab binds with TNF-alpha and inhibits its binding with p55 and p75 cell surface TNF receptors. In the presence of complement, adalimumab also lyses surface TNF expressing cells. Finally, adalimumab also mediates the various biological responses that are regulated by TNF such as changes in the levels of adhesion molecules responsible for white blood cells migration. The net effect is that inflammation in the body is reduced.
What are the indications of adalimumab?
Adalimumab can be given in the following diseases:
- Rheumatoid Arthritis
- Juvenile Idiopathic Arthritis
- Psoriatic Arthritis
- Ankylosing Spondylitis
- Adult Crohn’s Disease
- Pediatric Crohn’s Disease
- Ulcerative Colitis
- Plaque Psoriasis
- Hidradenitis Suppurativa
- Uveitis
What are the contraindications of adalimumab?
Patients treated with adalimumab are at an increased risk of developing infections. Therefore do not initiate adalimumab in patients suffering from active infections. Use adalimumab with caution in the following individuals
- with chronic or recurrent infection
- history of tuberculosis or any other opportunistic infection
- who have lived or traveled in areas where tuberculosis or mycoses is endemic
- those older than 65 years and with co-morbidities or taking concomitant immunosuppressants
What are the warnings and precautions associated with the use of adalimumab?
Serious infections
Adalimumab is an immunosuppressive drug therefore it will predispose its user to infections. Some of these infections can be life-threatening. Similarly it can cause re-activation of tuberculosis and hepatitis B. Therefore close monitoring of the patient for these infections particularly, and for all infections generally is needed.
Malignancies
All users should be warned about the possibility of developing cancer.
Heart failure
Adalimumab has not been formally studied in patients with heart failure, however, in clinical trials of other TNF blocker drugs it was seen there was worsening of pre-existing congestive heart failure. There were some cases of new onset CHF as well. Therefore close monitoring is recommended in patients with history of CHF or those at risk.
Autoimmunity
Autoantibodies develop in 3% to 26% of the people using adalimumab. The clinical consequence of developing these antibodies is variable. In some individuals these new antibodies do not cause any harm. However in some rare cases lupus-like syndrome develops. In such patients positive ANA titers might also be positive.
Neurological reactions
Anti-TNG drugs are associated with new onset or worsening of CNS demyelinating diseases such as multiple sclerosis and peripheral demyelinating diseases such as Guillain-Barre Syndrome. Caution must be observed when prescribing adalimumab to patients with new onset or pre-existing central or peripheral nervous system demyelinating disorders. Discontinuation of adalimumab should also be considered in such cases.
Hematological reactions
Aplastic anemia and blood dyscrasias have been noted with the use of adalimumab. Advise the patient to immediately report to the hospital if he has persistent fever, bruising, bleeding or pallor.
Pharmacokinetics of adalimumab
Route of administration: Subcutaneous injection
Absorption: After subcutaneous injection, the time to reach maximum plasma concentration was variable and ranged from 75 hours to 187 hours. The bioavailability of the drug is 64%.
Distribution: The volume of distribution of adalimumab ranges from 4.7 to 6.0 l. The amount of drug that reaches the synovial fluid is variable and ranges from 31% to 96% of the plasma drug concentration.
Clearance and half-life: The clearance of adalimumab is increased when anti-adalimumab antibodies are present and it is decreased with increasing age in patients aged 40 to >75 years. The half-life of adalimumab ranges from 10 to 20 days.
How is adalimumab stored?
Optimum temperature for storage of adalimumab ranges from 2°C to 8°C (36°F to 46°F) and do not freeze it. Store it in its original containers to protect it from sunlight. If it is stored at room temperature use it within 14 days. Failure to use it in that time period warrants that it be discarded.
Side effects of adalimumab
The following side effects have been seen with adalimumab
- Serious infections
- Malignancies
- CNS: Headache, confusion, paresthesia
- Skin: Rash, skin infections
- Local injection site: Erythema, itching, hemorrhage, pain, swelling
- Respiratory system: Upper respiratory tract infection, sinusitis, pneumonia, tuberculosis
- Cardiovascular system: Hypertension, arrhythmia, atrial fibrillation, cardiac arrest, chest pain, deep vein thrombosis, pericarditis, peripheral edema, syncope, hypertensive encephalopathy
- Gastrointestinal system: nausea and vomiting, diarrhea, abdominal pain, GI bleed, gall stones, hepatotoxicity
- Genitourinary: Urinary tract infection, blood in urine
- Hypersensitivity reaction
Adalimumab dosage
The recommended dosage of adalimumab in various diseases is as follows:
- Rheumatoid Arthritis: 40 mg every other week. Other DMARDs particularly methotrexate has been found to be beneficial in inducing a response. If monotherapy is being given, then raise it to 40 mg every week.
- Psoriatic Arthritis: 40 mg every other week. Combining it with methotrexate has been found to be beneficial in inducing a response. If monotherapy is being given, then raise it to 40 mg every week.
- Ankylosing Spondylitis: 40 mg every other week. If monotherapy is being given, then raise it to 40 mg every week.
- Adult Crohn’s Disease: For treating an acute attack, give 160 mg on day 1 followed by 80 mg on day 15. Maintenance dose of 40 mg is started on day 29 and given every other week.
- Ulcerative Colitis: For treating an acute attack, give 160 mg on day 1 followed by 80 mg on day 15. Maintenance dose of 40 mg is started on day 29 and given every other week.
- Plaque Psoriasis: 80 mg initial dose. Maintenance dose of 40 mg is given every other week starting one week after the initial dose.
- Hidradenitis Suppurativa: For treating an acute attack, give 160 mg on day 1 followed by 80 mg on day 15. Maintenance dose of 40 mg is started on day 29 and given every other week.
- Uveitis: 80 mg initial dose. Maintenance dose of 40 mg is given every other week starting one week after the initial dose.
Adalimumab off-label use
Pyoderma gangrenosum
Preliminary data suggest that adalimumab could be successful as an adjunct therapy or monotherapy for the treatment of severe pyoderma gangrenosum. Adalimumab has been shown to be beneficial in refractory pyoderma gangrenosum in cases in which infliximab and etanercept have failed. Controlled studies are needed to evaluate the efficacy and safety of adalimumab in order to fully determine its place in the treatment of pyoderma gangrenosum.
Uveitis (children/adolescents)
Results from noncontrolled data show that adalimumab is effective in treating uveitis in children and adolescents. However, no prospective controlled clinical trials have been performed. Adalimumab has several safety concerns, including a black box warning regarding serious infections and lymphomas.
Further data are needed to establish the efficacy, safety, optimal dosage, and length of adalimumab therapy in the treatment of uveitis in children. An expert review panel recommends adalimumab as a third-line treatment option in children with noninfectious uveitis.
Adalimumab price and prescription
Adalimumab is one of the most expensive drugs around. The average retail price of 2 pens of 40 mg (0.8ml) adalimumab is $5,384. GoodRx, the online retail pharmacy, offers a discount of 9% and it is available for $4,846 from there.
Can I take adalimumab if I am pregnant?
Adalimumab actively crosses the placenta, especially in the third trimester. This raises the possibility of suppression of immune system of the baby, meaning that the newborn would be at a higher risk of developing infections than other babies. Another problem in such newborns and infants is that whether vaccination should be carried out or not.
A study to determine the incidence of major birth defects when a woman takes adalimumab during pregnancy was also conducted in the US and Canada. The study found that the incidence was at 5.6%. This was not a statistically significant result as the estimated background risk of major birth defects is 2-4% in the US.
Although this study suggests that there is no added risk to the mother or fetus due to adalimumab, more research needs to be carried out to confirm this conclusion. Till then use of adalimumab during pregnancy should be judicious and on physician’s discretion.
Can I take adalimumab if I am breastfeeding?
The amount of adalimumab that is present in breast milk is small and ranges from 0.1% to 1%. Although there are no reports of side effects of adalimumab on the baby or on milk production, but still no clear cut go-ahead has been given to continue to breast feed the baby while taking adalimumab.
Therefore a physician should consider the developmental and health benefits of breastfeeding to the baby along with the mother’s clinical need and possible side effects before making a final decision.
Is adalimumab safe for children?
Safety and efficacy of adalimumab was carried out in children suffering from juvenile rheumatoid arthritis and juvenile Crohn’s disease. It was found that the drug had equal efficacy in children when compared with the efficacy in adults. The safety of the drug was also comparable in the two age groups however the profile of adverse effects was different in children.
Therefore it can be concluded that adalimumab can be given to children with juvenile rheumatoid arthritis or juvenile Crohn’s disease but not for any other disease as its safety for any other disease has not been studied.
Is adalimumab safe for elderly?
519 patients with rheumatoid arthritis aged 65 years or above were treated with adalimumab and the safety and efficacy of the drug was noted. Overall there was no difference in the efficacy of the drug in the elderly when compared with the younger age group. However the incidence of serious life-threatening infections and malignancies was higher in the elderly.
Therefore use of adalimumab in the elderly still remains a gray area and can only be decided after weighing the pros against the cons. Dose adjustment may also be needed in the elderly as its clearance is reduced in this age group due to decreased kidney function.
Does adalimumab cause infertility?
There is very little data available on the effect of adalimumab on the fertility of both males and females. The data available for other anti-TNF drugs suggests that anti-TNF agents do not cause infertility nor do they affect the outcome of pregnancy.
However since these drugs were made commercial only at the end of last century, and because their use is still limited, avoid the aggressive use of adalimumab in individuals wishing to preserve their fertility.
Can adalimumab cause cancer?
Every day a number of mutations occur in our cells, however our healthy immune system kills these mutated cells. Therefore if we suppress our immune system, the probability of developing cancer raises by many folds. The most common type of malignancies reported were lymphomas (both Hodgkin’s and non-Hodgkin’s lymphoma) and leukemia’s. The most common type of solid tumors were cancers of breast, colon, prostate, lung, and melanoma.
The risk is increased in all age groups and is directly proportional to the duration of intake of immunosuppressants as well history of use of other immunosuppressants as well such as azathioprine.
What are some drugs that can interact with adalimumab?
Adalimumab reacts with a number of drugs. Some of the drugs having a major interaction with adalimumab are:
- Biological products: Patients simultaneously taking adalimumab with other biological products such as anakinra or abatacept were at an increased risk of developing serious infections. Combined use of adalimumab with rituximab also presented with same result. Therefore administration of adalimumab with these drugs as well as other DMARDs in not recommended.
- Abatacept: Anti-TNF Agents may enhance the adverse/toxic effect of Abatacept. An increased risk of serious infection during concomitant use has been reported. Avoid combination
- Anakinra: Anti-TNF Agents may enhance the adverse/toxic effect of Anakinra. An increased risk of serious infection during concomitant use has been reported. Avoid combination
- BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
- Belimumab: Monoclonal Antibodies may enhance the adverse/toxic effect of Belimumab. Avoid combination
- Canakinumab: Anti-TNF Agents may enhance the adverse/toxic effect of Canakinumab. Specifically, the risk for serious infections and/or neutropenia may be increased. Avoid combination
- Certolizumab Pegol: Anti-TNF Agents may enhance the immunosuppressive effect of Certolizumab Pegol. Avoid combination
- Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
- CycloSPORINE (Systemic): Adalimumab may decrease the serum concentration of CycloSPORINE (Systemic). Monitor therapy
- Cytochrome P450 substrates: During chronic inflammation, increased levels of cytokines suppress the activity of P450 enzymes. Since adalimumab antagonizes cytokine activity, it indirectly influences the activity of P450 enzymes. Therefore patients taking drugs metabolized by these enzymes and having a narrow therapeutic index should be closely monitored and if needed, dose should be adjusted.
- Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
- Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
- Fingolimod: Immunosuppressants may enhance the immunosuppressive effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and other immunosuppressants when possible. If combined, monitor patients closely for additive immunosuppressant effects (eg, infections). Consider therapy modification
- InFLIXimab: Adalimumab may enhance the immunosuppressive effect of InFLIXimab. Avoid combination
- Leflunomide: Immunosuppressants may enhance the adverse/toxic effect of Leflunomide. Specifically, the risk for hematologic toxicity such as pancytopenia, agranulocytosis, and/or thrombocytopenia may be increased. Management: Consider not using a leflunomide loading dose in patients receiving other immunosuppressants. Patients receiving both leflunomide and another immunosuppressant should be monitored for bone marrow suppression at least monthly. Consider therapy modification
- Methotrexate: Concomitant use of methotrexate with adalimumab will reduce the apparent adalimumab clearance. However data collected does not suggest that there is a need for dose adjustment
- Natalizumab: Immunosuppressants may enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Avoid combination
- Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
- Ocrelizumab: May enhance the immunosuppressive effect of Immunosuppressants. Monitor therapy
- Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
- Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
- Rilonacept: Anti-TNF Agents may enhance the adverse/toxic effect of Rilonacept. Avoid combination
- Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
- Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Monitor therapy
- Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
- Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
- Theophylline Derivatives: Adalimumab may decrease the serum concentration of Theophylline Derivatives. Exceptions: Dyphylline. Monitor therapy
- Thiopurine Analogs: Anti-TNF Agents may enhance the adverse/toxic effect of Thiopurine Analogs. Specifically, the risk for T-cell non-Hodgkin’s lymphoma (including hepatosplenic T-cell lymphoma) may be increased. Monitor therapy
- Tocilizumab: May enhance the immunosuppressive effect of Anti-TNF Agents. Avoid combination
- Tofacitinib: Anti-TNF Agents may enhance the adverse/toxic effect of Tofacitinib. Avoid combination
- Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
- Vaccines (Inactivated): Immunosuppressants may diminish the therapeutic effect of Vaccines (Inactivated). Management: Vaccine efficacy may be reduced. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant. If vaccinated during immunosuppressant therapy, revaccinate at least 3 months after immunosuppressant discontinuation.
How long does adalimumab stay in your system?
The half-life of adalimumab is very long and ranges from 10 days to 20 days. Normally, 5.5 half-lives are needed to remove a drug from the body. Therefore adalimumab will be completely removed from an individual’s system after 55 days to 110 days. Presence or absence of other factors such as kidney function, age, concomitant use of methotrexate will also affect the half-life of adalimumab and may increase the duration that this drug stays in our system.