Contents
- What is doxepin?
- Doxepin description, IUPAC name, molecular formula, weight, structure, and class
- Doxepin identification
- What are the different dosage form and strengths available for doxepin?
- What are the indications for using doxepin?
- What is the mechanism of action of doxepin?
- What are the pharmacokinetics of doxepin?
- What is the recommended dosage of doxepin?
- Is there any consideration to be made in doxepin dosing?
- How is doxepin administered?
- What are the possible side effects of using doxepin?
- Doxepin use during pregnancy
- Doxepin use during breastfeeding
- Doxepin use in geriatrics
- Doxepin use and suicidal thoughts
- Doxepin and seizure disorder, electroconvulsive therapy (ECT)
- Doxepin and sleep apnea
- Doxepin and diabetes
- Doxepin and pheochromocytoma
- Doxepin anticholinergic effects
- Doxepin and risk of seizures
- Doxepin and glaucoma
- Doxepin and bone marrow transplantation
- Doxepin withdrawal
- Doxepin and MAO inhibitors interaction
- Doxepin and methylene blue interaction
- Doxepin and azithromycin interaction
- Doxepin and methadone interarction
- Can I take doxepin with alcohol?
What is doxepin?
Doxepin is a member of a class of drugs known as tricyclic antidepressants (TCAs). It is responsible for increasing the number of neurotransmitters which convey the messages in the brain.
These neurotransmitters include serotonin and norepinephrine. It has demonstrated a range of pharmacological actions yet its exact mechanism of action is still not known.
This drug is a prescription only product. Doxepin was approved by Food and Drug Administration in 2010.
Doxepin description, IUPAC name, molecular formula, weight, structure, and class
This drug is from a class of psychotherapeutic drugs distinguished as dibenzoxepin tricyclic compounds.
It shows a resemblance to the structure of phenothiazines in a way that these tricyclic antidepressants also contain a ring system containing an alkylamine substituent present on the central ring.
Doxepin is a tertiary amine which is more potent when compared with secondary amine tricyclic antidepressants.
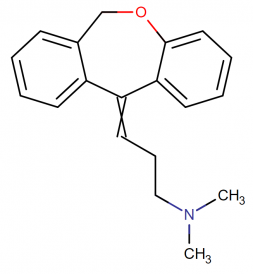
IUPAC Name: (3E)-3-(6H-benzo[c][1]benzoxepin-11-ylidene)-N,N-dimethylpropan-1-amine
Molecular Formula: C19H21NO
Molecular weight:
The average weight is 279.376
The weight of monoisotopic form is 279.162
The weight of HCl salt is 315.84
Molecular structure:
Drug class: Doxepin belongs to the class of organic compounds known as dibenzoxepines. These are compounds containing a dibenzoxepine moiety, which consists of two benzene connected by an oxazepine ring.
Doxepin identification
It is commonly used as an HCl salt. This compound is a white crystalline solid which shows high solubility in water, chloroform and low molecular weight alcohols.
What are the different dosage form and strengths available for doxepin?
Doxepin is available in the following forms:
The capsule is usually available by the generic name. It is manufactured in the following strengths:
- 10mg
- 25mg
- 50mg
- 75mg
- 100mg
- 150mg
The tablet is available by generic and brand name both. It is manufactured in the following strength:
- 3mg
- 6mg
The oral concentrate is available by only generic name. It is prepared in the strength of 10mg/ ml
The topical cream is available in the strength of 5%. This is available by generic and brand name both.
What are the indications for using doxepin?
The oral dosage form is indicated for use in the following conditions:
- It is used in the treatment of depression and/ or anxiety.
- It is used in the treatment of depression linked with the use of alcohol
- It is used in the treatment of depression/anxiety that is associated with any organic disease.
- It is used in the treatment of insomnia typically in which disturbances occur with maintained sleep period.
The topical dosage form of doxepin is:
- Indicated for a short-term treatment and management of mild to moderate pruritis in an adult patient with lichen simplex chronicus and atopic dermatitis.
What is the mechanism of action of doxepin?
The mechanism of action of doxepin is still not fully understood but generally it is thought to that their most of the action is due to the effect of enhanced role or serotonin (5-HT) and norepinephrine.
This is done by blocking the reuptake of a variety of different neurotransmitters at the neuronal membrane.
Doxepin has a mechanism of action which is similar to the way amitryptiline exerts its action: both drugs act on serotonin and norepinephrine.
According to some recent evidence studies it has been observed that the disturbed monoamine output in patients suffering from depression and/ or anxiety can be normalized when antidepressants are given for a chronic duration.
The suggested reason behind this is that antidepressants act on the beta adrenergic receptors and this is possibly a better explanation than the reuptake of these neurotransmitters by these drugs.
When doxepin is used in the treatment and maintenance of insomnia or even for regulating the disturbed sleep cycle, they act on the H-1 receptors and antagonize its function.
Tricyclic drugs do not act on monoamine oxidase neither they show any action for the reuptake of dopamine neurotransmitters.
Sedation of variable intensities can be produced from using doxepin due to its strong affinity of binding with the histamine H-1 receptors which ultimately lowers the seizure threshold.
The antipruritic activity of doxepin is considered secondary to the obstruction of histamine H-1 receptor.
Furthermore, the antagonist action on cholinergic recpetors is only to a moderate level and is the reason for which the inhibition of urination occurs thus effectively used in the treatment of enuresis.
Action on the heart such as cardiac dysarythmias can take place due to the quinidine like action which directly acts on heart muscles along with the anticholinergic activity and enhanced norepinephrine potentiation.
In addition to all these possible ways in which doxepin can act alterations in the concentrations of sex hormones and blood glucose level are also seen.
What are the pharmacokinetics of doxepin?
Doxepin is the dosage form which is given to the patient via the oral route and through topical application. It has shown a wide distribution pattern in the body.
It has been studied that the active metabolite is also found in the breast milk of humans. The maximum and complete antidepressant action of doxepin can take at least up to 2 weeks or even more in some cases to stabilize the condition of the patient.
On the other hand the adverse effects that take place after the administration of the drug can be observed within a few hours after the first dose.
Doxepin has shown approximately 80% binding with the plasma proteins especially albumin. Doxepin is a long acting anti depressant which makes it beneficial over other antidepressants.
Administration of a single dose can be given to enhancing the patient compliance.
This drug is majorly metabolized by CYP2C19 and CYP2D6 and a minute portion is metabolized by CYP1A4 and CYP2C9.
In some of the patients about 7 – 10% of the population of who is using this drug is genetically poor metabolizers as they have shown decreased activity of CYP2D6 or even CYP2C19.
These poor metabolizers are more prone to suffer from adverse reactions.
As doxepin is metabolized by these hepatic isozymes, all those drugs that will inhibit or promote the action of these isozymes will also affect the metabolism of doxepin.
In the metabolism of doxepin, the major metabolite that is formed is N-desmethyldoxepin which is also known as nordoxepin.
When these metabolites are further glucoronidated, the resulting product in combination with the parent drug is excreted through the body via urine.
The estimated half-life of doxepin is between 6-8 hours whereas the half life for desmethyldoxepin ranges from 28 – 52 hours.
It has been observed that the half life of the active metabolite is not affected by giving the drug for prolonged periods.
Doxepin undergoes extensine metabolism and only a small portion of the drug is excreted either in form of active metabolite nordoxepin or in form of unchanged drug doxepin.
Oral Route:
Oral dosage forms of doxepin have shown a good absorption from the gut. The the time to reach mean plasma peak concentrations after oral doxepin is ingested is approximately 3.5 hours when the pasting is in fasting condition.
Giving doxepin with meals containing high fat content raises the AUC by 41% and the maximum plasma concentrations (Cmax) by 15%. Moreover, the tmax is delayed by 3 – 4 hours.
Due to this reason, the time gap in between the intake of fatty meals and doxepin should be round about 3 hours. Following a dose of 6mg of doxepin, the mean apparent volume of distribution was 11,930L.
Topical route:
On applying doxepin topically, doxepin is absorbed in the blood circulation via the skin. Percutaneous absorption produces the plasma concentrations which are highly variable and ranges from such small amounts which are non-detectable to 47mg/ ml.
This percutaneous absorption in increased to a great extent when occlusive dressings are used. The plasma concentrations which are obtained by using doxepin topically are high enough to cause various systemic and CNS side effects.
What is the recommended dosage of doxepin?
Doxepin should be given to the patients in a dose which is individualized according to the disease condition.
- In the treatment of the depression/ anxiety also psychotic depressive disorders linked with anxiety:
(Oral route):
In adults:
The starting dose should not be more than 75mg/ day PO which can be given as a single dose at bedtime or can be administered in 2 to 3 divided doses. The dose of doxepin can be gradually raised depending on the clinical response and tolerability of the patient.
In geriatric patients it is recommended to the start the dose at a low range, and slowly and at regular intervals titrate the dose.
Keep a close check on the patient to observe the appearance of any unwanted cholinergic effect. The patient should be monitored for oversedation also.
Normal dose range of doxepin is 75 to 150mg/day PO (can be given as a single dose or in divided doses).
Patients having mild symptoms can be given 25 – 50 mg/day PO
In severely ill patients, higher doses of maximum 300 mg/day PO can be given. (it is advised that when doses greater than 150 mg/day should be given in divided doses).
In adolescents:
In adolescents safety profile and efficacy studies have yet not been established but when the treatment is started with doxepin it should be started with the lowest dose and gradually titrate it at regular weekly intervals.
Even though tricyclic antidepressants are not recommended as a drug of choice in adolescents who are suffering from depression but the doses based on weight have been standardized as:
Normal dose is 1mg/kg/day to a maximum of 3mg/kg/day (in single or divided doses) which is about 25 mg/day to 50 mg/day PO.
In adolescents, it should not exceed 100 mg/day PO.
- In the treatment of insomnia typified by problems with sleep maintenance:
(Oral route):
In adults:
Doxepin 3mg PO should be given once a day within 30 – 40 minutes of bedtime which according to the need of the patient can be increased upto 6mg PO once a day within 30 – 40 minutes of bedtime. The dose should not be more than 6 mg/day PO.
In geriatrics:
In geriatrics, patients have less metabolizing ability thus it is suggested to administer doxepin at a lower dose of 3 mg/kg in 30 minutes of bedtime. When it is clinically indicated the dose of doxepin can be increased upto a maximum of 6 mg/day PO.
- In the treatment of eczematous dermatitis (atopic dermitits, lichen simplex complex and eczema)
(Topical application – 5% cream):
In adults:
On the affected area, apply the cream on the skin 4 times in a day, keeping a gap of 3 – 4 hours between two consecutive applications.
This should be continued for upto 8 days. Safety profile has not yet been established for using the cream for more than 8 days.
If the topical application of doxepin leads to sedation, the frequency of dexepin application can be reduced or the quantity of cream applied or even the drug can be discontinued in some cases.
- In the prophylaxis of a migraine: (this is an off-label use)
(Oral route):
In adults:
For migraine prophylaxis dose range of 10 mg/day to 150 mg/day can be administered to the patient. In one of the literature, it was published an optimal dose of 50 mg/kg was used for this purpose.
However, doxepin is still being studied and still has to be included in present guidelines.
Is there any consideration to be made in doxepin dosing?
In patients who show signs of hepatic impairment, it is recommended to adjust the dose accordingly.
No guidelines have yet been established for the topical preparations but for oral dosage forms such as capsules and oral concentrate solutions, the starting dose should be reduced to such a level until a required clinical response or tolerability among the patients is not obtained.
While prescribing tablets, the lower dose of doxepin that is 3mg should be prescribed to the patient and a close check for any unwarranted effects should be kept.
As doxepin is extensively metabolized and only 3% of the drug either in form of metabolite or unchanged parent drug or both is excreted through urine so no special modifications are made in patients who have impaired renal function.
How is doxepin administered?
Each dosage form of doxepin is carefully administered in order to maximize its availability and hence effect for the patient.
Dosage forms which are administered via oral route:
- Oral Solid Preparations:
- Capsules: the major effect that takes place from using tricyclic antidepressants is sedation and sleep. To reduce this effect during the daytime, doxepin capsules as a single dose should be administered at bedtime.
- In some of the patients, on using doxepin they suffer from insomnia. They should thus use the medicine in the morning time.
- Tablets: doxepin tablets are advised to only take just before bedtime. In order to lower the possibility for next day effects, it should not be administered within 3 hours of a meal.
- Oral Liquid Preparations:
Oral concentrated solutions are required to be diluted before use. Prior to administration every single dose should be diluted with 120mL of any fluid such as water, juices for example pineapple, orange, tomato, grapefruit or prune, skimmed or whole milk.
Caution should b taken on not diluting the oral concentrate with any type of carbonated beverages. Preparation should not be prepared in bulk. Each time a new dose of doxepin should be prepared.
Note: when doexpin is given in combination with methadone, methadone syrup and doxepin solution can be together mixed with water, sugar water, orange juice or lemonade.
Dosage forms which are applied topically to skin:
- Lotion/ Cream or Ointment Preperations:
These preparations are topically applied to skin only.
Caution should be taken to not to apply to the eye or intravaginally or administered via the mouth. Carefully rub the cream onto the affected areas.
Avoid using any occlusive dressing or bandage.
What are the possible side effects of using doxepin?
Commonly occurring side effects of using doxepin are listed below:
- Feeling tired, dizzy or drowsiness
- Nausea or vomiting
- Constipation
- Tinnitus (ringing in the ears), dry mouth or blurred vision
- Weight gain, enhanced sweating
- Breast swelling in both male and women
- Libido that decreased sex drive.
More serious side effects which include:
- An allergic reaction which includes skin reactions, difficulty in breathing, swelling of the face, lips and tongue.
- Mood or behavior changes
- Anxety or panic attacks in which the patient feels agitated, aggressive, hostile, hyperactive or may become more depressed having suicidal thoughts.
- Seizures(convulsions), tremors restless muscle movements
- Cardiac ventricular arrhythmias
- Pain and difficulting in passing urine
- Ocular hypertension
- Syndrome of inappropriate anti-diuretic hormone (SIADH)
In a case where any of these serious unwanted side effects take place, the patient should be immediately taken to the emergency in order to get treatment.
Doxepin use during pregnancy
Doxepin and other tricyclic antidepressants belong to pregnancy category class C.
As sufficient data are not available so it is advised to only prescribe the drug when the clinical benefits are dominant over the potential side effects for the mother and the fetus both.
Doxepin has shown penetration across the placental barrier. Neonatal problems have been observed on administrating doxepin to the mother.
These complications include hypoglycemia, respiratory problems, malformations, jaundice and delay in the development of bodily organs and systems. These neonates who are exposed to doxepin should be monitored regularly until birth.
Doxepin use during breastfeeding
According to the claims made by some of the manufacturers of oral dosage forms, doxepin is distributed in the breast milk.
According to a case report, unwanted side effects have been observed in infants who had been exposed to doxepin from the breast milk of the mother.
These adverse effects included apnea and drowsiness more commonly and seizures to less extent.
In infants whose mother’s were on a dose of 35 mg/day had to suffer from vomiting, weight loss, muscle hypotonia and poor sucking and swallowing abilities.
The degree of exposure to the breast fed infant from doxepin at a lose dose is still unknown so it is not recommended to breastfeed the child while the mother is taking doxepin.
Infants have a less metabolizing capability, so the transfer of doxepin from the milk will adversely affect the baby’s system and bring about unwanted effects.
Doxepin use in geriatrics
The clinical outcomes on the administration of tricyclic antidepressants vary among different age groups. While administrating doxepin to geriatric, caution must be taken to as their body systems and organs functional ability has been reduced with age.
It is recommended that the starting dose should always be the smallest dose possible; depending upon the degree of the medical condition for which the drug is prescribed the dose can then be gradually titrated.
As the increments in dosage are made in such patients, careful observation is necessary to prevent them from suffering any adverse effect.
Generally it is observed that sedation and confusion are present in such individuals. On using doxepin cream, in some of the elderly patients urinary retention and delirium are reported.
Depending on the amount of doxepin cream, it has also caused dementia and cognitive imapirement and in men prostatic hyperplasia is seen on chronic use of doxepin cream.
Doxepin use and suicidal thoughts
Children who are below 12 years of age should not be prescribed these antideprassants for the treatment of depression.
The topical application is not even recommended in peadritics possible due to poor absorption and metabolism power of their bodies.
FDA has issued a warning for the use of these drugs as they induce suicidal thoughts among children.
Besides having suicidal thoughts, children were more aggressive and shown unusual behavior changes especially during the intial days when the treatment was started.
Doxepin and seizure disorder, electroconvulsive therapy (ECT)
All those patients are already suffering from any kind of seizure disorders, doxepin should be used with extreme caution in such patients as these tricyclic antidepressants agents can lower the seizure threshold.
If the seizure occurrence rate increases on use with doxepin, the drug should be discontinued.
Furthermore, the coadministration of doxepin with the electroconvulsive therapy increases the dangers that are associated with the therapy. So this should only be limited in who it is necessary and no other alternative is available.
Doxepin and sleep apnea
Doxepin is also used in treating rebound insomnia but still, studies are being conducted on use by patients who are suffering from obstructive sleep apnea.
As doxepin is a hypnotic agent it has the capacity to depress the respiratory system, so caution is required if this drug is given to patients who have impaired respiratory function for the treatment of insomnia.
In patiets who are going through severe sleep apnea, doxepin should be avoided it may worsen up the condition.
Doxepin and diabetes
Both elevation and lowering of blood sugar levels have been reported with the use of some tricyclic antidepressants (TCAs) including doxepin.
Rarely, these effects have also occurred with maprotiline, a tetracyclic antidepressant. Patients with diabetes should be monitored for worsening control of blood glucose when treated with these agents, particularly during dosage escalation or whenever dosage has been altered.
Doxepin and pheochromocytoma
Tricyclic and tetracyclic antidepressants (TCAs) may potentiate the effects of circulating catecholamines.
Enhanced sympathetic activity can provoke hypertensive crises in patients with pheochromocytoma or other tumors of the adrenal medulla, such as some neuroblastomas.
Therapy with TCAs should be administered cautiously in patients with these tumors.
Doxepin anticholinergic effects
Tricyclic and tetracyclic antidepressants (TCAs) such as doxepin have anticholinergic activity, to which elderly patients are particularly sensitive.
Tertiary amines such as amitriptyline and trimipramine tend to exhibit greater anticholinergic effects than other agents in the class.
Therapy with TCAs should be administered cautiously in patients with preexisting conditions that are likely to be exacerbated by anticholinergic activity, such as urinary retention or obstruction; angle-closure glaucoma, untreated intraocular hypertension, or uncontrolled primary open-angle glaucoma; and gastrointestinal obstructive disorders.
In patients with angle-closure glaucoma, even average doses can precipitate an attack. Glaucoma should be treated and under control prior to initiation of therapy with TCAs, and intraocular pressure monitored during therapy.
Doxepin and risk of seizures
Tricyclic antidepressants (TCAs) such as doxepin, can lower the seizure threshold and trigger seizures.
These drugs should be used with extreme caution in patients with a history of seizures, or other predisposing factors, such as head trauma, CNS abnormalities, and alcoholism.
Daily dose restrictions might apply for specific antidepressants. Physicians are encouraged to get additional dosing recommendations on the manufacturer’s prescribing information.
Doxepin and glaucoma
Doxepin may exhibit anticholinergic effect and should be used with caution in patients with glaucoma, and trouble urinating due to retention or enlarged prostate.
Doxepin and bone marrow transplantation
The use of tricyclic and tetracyclic antidepressants (TCAs) including doxepin has rarely been associated with bone marrow suppression.
Leukopenia, agranulocytosis, thrombocytopenia, anemia, eosinophilia, purpura, and pancytopenia have been reported with some TCAs.
Patients with preexisting bone marrow suppression or blood dyscrasias receiving TCAs should be monitored closely during therapy for further decreases in blood counts.
Doxepin withdrawal
Doxepin should never be abruptly stopped as it may worsen up a drug discontinuation syndrome. This is characterized by symptoms which are typical of cholinergic rebound effects such as diarrhea, nausea, and vomiting.
More serious symptoms include hyperarousal, sensory disturbances, imbalance and flu-like symptoms. This withdrawal symptom has more chances to occur when doxepin is used at a low dose.
Doxepin and MAO inhibitors interaction
Using doxepin in combination with monoamine oxidase inhibitors (MAOI) is not recommended. It is suggested to not to even use doxepin topically during the therapy with MAOI or even within 14 days the monoamine oxidase inhibitors have been discontinued.
The exact delay that is required between these two drugs depends on the dasage involved, the route of administration and the course of therapy for which it was prescribed.
Even when doxepin is applied topically, the plasma levels of the drug reach to a concentration that can bring about interactions with even a small quantity of MAOI present in the blood.
Doxepin and methylene blue interaction
Patients who are already receiving intravenous methylene blue, the administration of doxepin interact and produce a strong serotonin syndrome. When there is an urgent need for psychiatric treatment, drugs other than doxepin should be used.
In patients who are on a doxepin therapy and require an urgent administration of IV methylene blue, the tricyclic antidepressant should be immediately discontinued and the IV treatment should start.
The patient should be closely monitored for a minimum time period of 2 weeks after the most recent dose of methylene blue is administered. Symptoms typically include lethargy, confusion, myoclonus, elevated blood pressure and/ or coma.
Doxepin and azithromycin interaction
When azithromycin is administered concomitantly with doxepin, prolongation of QT interval and ventricular arrhythmias such as torsade de pointes.
Doxepin has some of the pharmacological properties which resemble class 1A antiarrhythmic thus prolonging QT interval. This is especially in cases of overdosage or high doses of the drug due to high plasma concentrations.
Doxepin and methadone interarction
Methadone may cause dose-related prolongation of the QT interval. Theoretically, coadministration with other agents that can also prolong the QT interval such as doxepin may result in additive effects and thus increased risk of ventricular arrhythmias including torsade de pointes and sudden death.
High dosages of methadone alone have been associated with QT interval prolongation and torsade de pointes.
In a retrospective study of 17 methadone-treated patients who developed torsade de pointes, the mean daily dose was approximately 400 mg (range 65 to 1000 mg) and the mean corrected QT (QTc) interval on presentation was 615 msec.
The daily methadone dose correlated positively with the QTc interval. Fourteen patients had at least one predisposing risk factor for arrhythmia (hypokalemia, hypomagnesemia, concomitant use of a medication known to prolong the QT interval or inhibit the metabolism of methadone, and structural heart disease), but these were not predictive of QTc interval.
It is not known if any of the patients had congenital long QT syndrome.
Can I take doxepin with alcohol?
Concomitant use of alcohol and a tricyclic antidepressant (TCA) such as doxepin may result altered TCA plasma levels and efficacy, and additive impairment of motor skills, especially driving skills.
Acute ethanol ingestion may inhibit TCA metabolism, while chronic ingestion of large amounts of ethanol may induce hepatic TCA metabolism.