Contents
- What is Alcohol?
- Mechanism of action of Alcohol:
- Alcohol dependence:
- Chemical and physical properties of Alcohol:
- Biological properties of Alcohol:
- Absorption of Alcohol
- Metabolism of Alcohol
- How long does Alcohol stay in your system?
- Alcohol Drug Testing Methods:
- How to minimize the Blood Alcohol Concentrations?
What is Alcohol?
Alcohol is a “depressant” drug involved in modulating thinking and behavior by affecting brain. Alcohol had been in use since ancient times and its use can be traced back to 8000 BC.
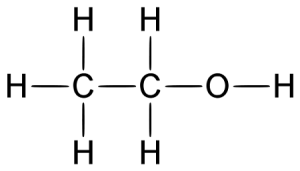
Alcohol belongs to the class of organic compounds known as primary alcohols. It is chemically known by other names such as ethanol; ethyl alcohol; methylcarbinol; grain alcohol; ethyl hydroxide.
Ethanol (alcohol) is a naturally occurring byproduct of the yeast metabolism and can also be commonly found in overripe fruit. Pure alcohol (Ethanol) was first discovered by Iranian/Persian alchemist named Muhammad ibn Zakariya al-Razi during wine distillation.
Alcohol is normally produced by the fermentation process, and also by distillation of various fruits, vegetables or grains. Fermented beverages such as beer and wine possess maximum alcohol content (about 15 per cent).
Distilled beverages, also known as “hard liquor” or “spirits,” (rum, whisky and vodka), also have high alcohol content. Alcoholic beverages are characterized by their distinctive colors arising due to their ingredients besides alcohol and the process of fermentation.
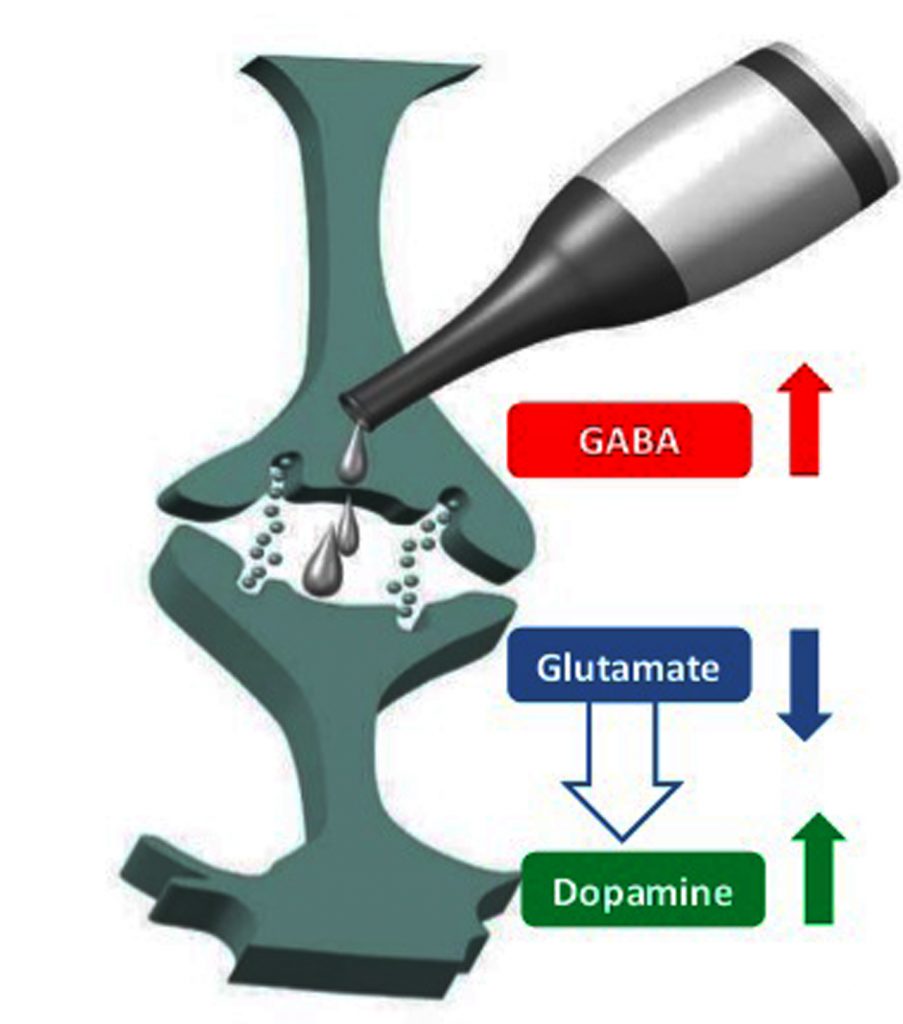
Alcohol can act as a central nervous system (CNS) depressant owing to its effect on the calcium channels mediated by gamma-amino butyric acid (GABA) -A receptors and N-methyl-D-aspartate (NMDA)-type glutamate receptors, a diuretic (via inhibition of antidiuretic hormone production), and also as a disinfectant due to its bactericidal activity.
Alcohol is the primary ingredient in alcoholic beverages and also used as a solvent and preservative in various pharmaceutical formulations. Alcohol is implicated in a variety of diseased states.
It is administered intravenously in patients for pre and post operative sedation where other measures do not work. It is also used as local anti-infective drugs. Alcohol (Ethanol) injections are given in orbit for relief of severe ocular or neuralgic pain.
Besides, dehydrated ethanol is injected in close proximity of nerves for relief of long lasting pain in patients with inoperable carcinoma, and other conditions.
Alcohol is also recommended for the treatment of methyl alcohol and ethylene glycol poisoning. Percutaneous intranodular ethanol injection (PNEI) is also prescribed for thyroid nodule treatment and for multiple hepatocellular carcinoma (HCC).
Mechanism of action of Alcohol:
What does alcohol do to neurotransmitters? Ethanol interaction with a variety of proteins including neurotransmitter-gated ion channels, and neurotransmitters contributes to its mechanism of action.
Ethanol causes enhancement of the effects of gamma-aminobutyric acid (GABA) at GABAa receptors and blockade of the N-methyl-D-aspartate (NMDA) subtype of glutamate (excitatory amine acid (EAA)) receptor.
NMDA receptors put forth its neurotoxic effect by increasing calcium permeability and regulating neuronal long-term potentiation. Ethanol competes with glycine for binding to the NMDA receptor and causes disruption of glutamatergic neurotransmission.
This results in the compensatory up-regulation of NMDA receptors. Ethanol tolerance causes increased EAA neurotransmission and NMDA receptor up-regulation. Abrupt Ethanol withdrawal results in hyperexcitable state causing Ethanol withdrawal syndrome.
Also, Ethanol administration can cause tolerance, dependence, and withdrawal syndrome via down-regulation of GABAa receptors and intensification of excitement.
Ethanol also causes an increase in dopamine release by inhibiting NMDA receptor activity and could thus create dependence. Ethanol also prevents adenosine uptake and causes an increase in synaptic adenosine concentrations thereby causing motor impairment.
Learned tolerance. It is also known as behaviorally augmented tolerance. The development of Alcohol tolerance gets accelerated by practicing a task while under the influence of Alcohol. Human beings also develop tolerance at a more rapid rate and at lower alcohol doses while practicing a task under the influence of Alcohol.
Environment-independent tolerance. It is developed under exposure to large quantities of Alcohol independently of environmental influences. Significantly larger amount of Alcohol is required for the development of this environment-independent tolerance in contrast to environment-dependent tolerance.
Metabolic Tolerance: It is developed due to more rapid rate of Alcohol elimination from the body. It is associated with a specific group of liver enzymes that are activated during chronic drinking and responsible for metabolizing Alcohol.
These enzymes cause an increase in Alcohol degradation and reduce the duration of Alcohol’s intoxicating effects.
However, the effect of these enzymes may also result in an increased metabolism of some drugs/medications including sedatives (e.g., barbiturates), drugs for prevention of blood clotting and treatment of diabetes and painkiller such as acetaminophen thereby causing harmful effects on the drinker.
Alcohol dependence:
What does it mean to be an alcoholic? Alcohol dependence, also known as alcoholism is a powerful driving force that develops in the brain and determines the drinking.
Factors contributing to Alcohol dependence include life style, difficult personal relationships, traumatic experiences from the past and underlying medical or mental health disorder.
The symptoms include craving, loss of control, physical dependence. Physical dependence on Alcohol involves tolerance to Alcohol’s effects, and occurrence of withdrawal symptoms when drinking is stopped.
An increasingly large amount of Alcohol is required to produce the desired effect by the people in case of development of tolerance. Physical dependence on Alcohol often elicits withdrawal symptoms, including sleeplessness, nausea, tremors and seizures, within a few hours after the last consumption of Alcohol. These symptoms can last for several days with varying severity.
Treatment for Alcohol dependence involves treatment of withdrawal symptoms, but in many cases an additional is needed. A person may carve Alcohol even after long periods of non consumption of Alcohol and may start taking it again.
Treatment can be done either at residential or community setting and may involve individual or group therapy, self-help or mutual help groups, and certain drugs (e.g., naltrexone). Some people may respond to one form of treatment, while others do not.
Chemical and physical properties of Alcohol:
- Alcohol belongs to the class of organic compounds known as primary alcohols and contains hydroxyl functional group, with the general structure RCOH.
- Alcohol is composed of carbon, oxygen, and hydrogen and is represented by the chemical formula C2H5
- It is a clear colorless liquid with characteristic odor which is mild rather pleasant; such as that of wine or whiskey.
- It has a burning taste.
- It is soluble in water and has a water solubility of 579.0 mg/mL.
- The melting point of Alcohol is -114.1 °C and boiling point is 78.2 °C.
- The pKa value of Alcohol is 15.9 (at 25 °C).
- The molecular weight of the compound is 46.06844 g/mol.
- Alcohol is miscible with many organic solvents including ethyl ether, acetone, chloroform and soluble in benzene.
- Alcohols undergo oxidation in the presence of an oxidizing agent to produce aldehydes and ketones which upon further oxidation give carboxylic acids.
- Upon treatment with strong acids, alcohols undergo dehydration (removal of a molecule of water) to form alkenes.
- Alcohols undergo complete combustion in excess oxygen resulting in the production of carbon dioxide and water with the liberation of energy.
- Alcohols react with active metals to produce a salt and hydrogen gas.
- Alcohols react with acids to produce esters.
Biological properties of Alcohol:
- Alcohol is readily absorbed in the body from the stomach and the intestine with the highest blood level reaching in about 30 minutes after the last drink.
- Alcohol absorption is decreased by the presence of food in the stomach but again accelerated once it reaches the small intestine.
- Alcohol vapors can be inhaled and get absorbed into the lungs which can cause serious industrial occupational hazard.
- Majority of the Alcohol after consumption and absorption distributes itself into body water while some distributes into fat.
- It is excreted in the urine and breath.
- The majority of Alcohol gets metabolized in the liver by enzymes alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase (ALDH).
- The depressant affects of Alcohol are attributed to its ability to dissolve cells’ lipid membranes and the disruption of the function of various proteins.
Absorption of Alcohol
Alcohol consumption is accompanied by the induction of some simultaneously occurring processes including absorption, distribution and metabolism (elimination).
Absorption is the passage of Alcohol into the bloodstream. Distribution is the process wherein Alcohol places itself temporarily into various body tissues whereas elimination is the removal of Alcohol from the body after being metabolized.
Alcohol is readily absorbed after oral administration via diffusion into the bloodstream from the stomach and small intestines resulting in its distribution throughout the body.
Large surface area as well as rich blood supply results in more rapid absorption of Alcohol from the small intestine in contrast to stomach and delay in gastric emptying causes slow absorption of Ethanol (Alcohol).
Various factors determine the rate of Alcohol consumption depending upon an individual’s physiological and biological factors. These include:
- Strength of Drink: Stronger drinks having higher Alcohol concentration generally cause higher Blood Alcohol Concentration (BAC).
- Gender: Males differ from female in metabolizing Alcohol more effectively due to presence of higher concentration of enzyme (dehydrogenase) responsible for breakdown of Alcohol, low fat in the body and hormonal difference.
- Body Weight: A bulky individual with higher water and blood level in body tends to have lower blood concentration of Alcohol in comparison to a smaller individual.
- High Stress vs. Relaxed: Stress results in emptying stomach directly into small intestine which absorbs Alcohol even much quickly. Decreased stress causes slowing down the rate of gastric emptying thereby delaying Alcohol absorption and reduction in the peak blood Alcohol concentration.
- Full vs. Empty Stomach: Food in the stomach generally prevents Alcohol from reaching the small intestine due to closure of the pyloric valve at the bottom of the stomach responsible for holding and digesting the food. Hence, larger meal and consumed more closely in time to drinking results in lowering of the peak of Alcohol concentration in the blood.
- Carbonation: Alcohol passage from the stomach to the small intestine is accelerated when Alcohol is mixed with soda or other bubbly drinks thereby causing an increased absorption rate.
- Medications: Some drugs, medications and over the counter medications can cause adverse effects and harmful interactions with Alcohol e.g., Acetaminophen causes significant liver complications in presence of Alcohol and Antihistamines when co-administered with Alcohol causes drowsiness and increased depressant effects of Alcohol. Hence, it is always recommended to consult a doctor for the possible use of the Alcohol with the drug/medication or over the counter medication.
- Health Concerns: Diabetes, depression, genetic enzyme deficiencies that can causes breakdown of Alcohol (alcohol dehydrogenase and aldehyde dehydrogenase), seizure disorder, hypertension, and other health issues may pose increased health risks by causing a decrease in the body’s ability to process Alcohol. Alcohol use may worsen these conditions and/or increase risk of developing chronic diseases.
Metabolism of Alcohol
How does the body break down alcohol? Most of the Alcohol (90% to 98%) that is consumed in the body is metabolized to acetaldehyde and then to acetate, primarily in the liver and only a small amount of it is excreted/eliminated unchanged in urine, sweat, feaces, milk, saliva and breath.
Elimination of Alcohol occurs at a constant rate for a given individual. Alcohol metabolism is relatively independent of its concentration in blood and is constant over time.
Although body weight determines the rate of metabolism, but on an average, Ethanol (Alcohol) is metabolized by the body (70 kg person) at a rate of 120 mg/kg per hour.
Ethanol is metabolized through the involvement of at least 3 different pathways including the alcohol dehydrogenase (ADH) pathway (located in the hepatocyte cytosol), the microsomal ethanol-oxidizing systems (MEOS or CYP2E1 located on the endoplasmic reticulum), and the peroxidase-catalase system (associated with the hepatic peroxisomes).
The ADH pathway represents the main pathway involved in the metabolism of Ethanol and is also the rate-limiting step. This pathway involves the enzyme alcohol dehydrogenase (ADH) that metabolizes Ethanol (Alcohol) to acetaldehyde which is toxic and associated with adverse effects such as flushing, headache, nausea, and vomiting.
Acetaldehyde in turn is rapidly metabolized to the less toxic metabolite acetate via cytosolic and mitochondrial aldehyde dehydrogenase (ALDH) in the liver. Metabolism is affected by a number of factors including food, gender, liver health and genetics.
How long does Alcohol stay in your system?
Alcohol enters into the body system slowly after being absorbed and remains in the blood until it is expelled by the body. After being absorbed in the bloodstream, Alcohol usually eliminated from the body in two ways: approximately 10% is expelled through normal excretory system in the form of breath, perspiration and urine, and the remainder is broken down through the process of metabolism which is independent of person’s size, weight, sex or race.
Local, state and federal laws have been created with the aim to safeguard public against undesirable Alcohol effects at workplace or while driving and require that one should submit to an Alcohol test when requested.
There are many factors that determine the duration of Alcohol stay in the body such as age, gender, metabolic rate, medications, food intake, time passed since last drink and type of Alcohol consumed.
Besides these, another important variable that determines exactly how long Alcohol can be detected in the system depends on the type of drug test. All these factors together make it almost impossible to determine an exact time of Alcohol detection in a drug test.
Alcohol Drug Testing Methods:
-
Breath Alcohol Test (BAT):
It is also known as breathalyzer test and measures the amount of ethyl alcohol via gas chromatographic procedure. Breath tests are more useful in determination of both the presence and the degree of acute Alcohol intoxication (a person with an Alcohol level of great than 0.08% is considered legally intoxicated).
Since, these tests are much simpler, faster, accurate and less invasive than blood tests, they are most commonly employed for the presence and amount of Alcohol consumed.
Breathalyzer tests are frequently employed by law enforcement agents for determining whether an individual is “over the legal limit” (0.08 BAC) for driving.
In this test an individual breathes into a breathalyzer, and Alcohol passes through alveolar sacs simultaneously with blood flowing through vessels within the lungs, and the Alcohol is detected through a person’s breath. The detection window for Alcohol in breathalyzer tests is up to 24 hours following consumption.
-
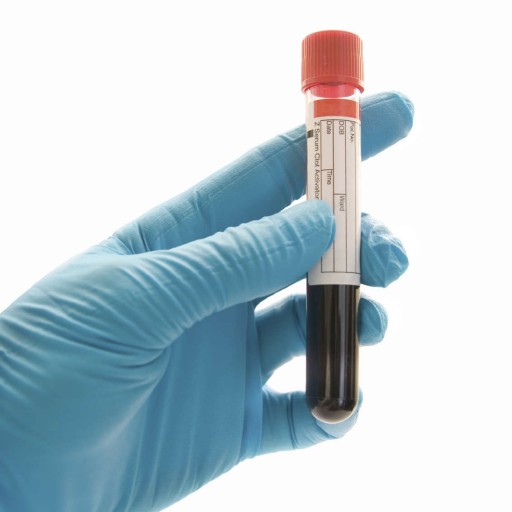
Blood Alcohol Test:
This is the preferred test in chronic alcoholism or in an acutely intoxicated patient. This test determines the presence and the amount of ethyl alcohol in the donor’s blood.
This test is usually performed utilizing approximately 4 ml of blood to assess if the tested individual is under the influence of Alcohol. Determination of the Alcohol concentration in blood requires measurement of the blood Alcohol concentration (BAC), which is read as a percentage and help in determining duration of Alcohol stay in body.
The rate at which Alcohol is metabolized and removed from the system by the human beings is about 0.15 of BAC per hour. Alcohol can be detected in the blood for up to 12 hours following a person’s last drink.
The detection window is dependent upon various parameters such as the amount of Alcohol ingested, sex and individual’s metabolic rate.
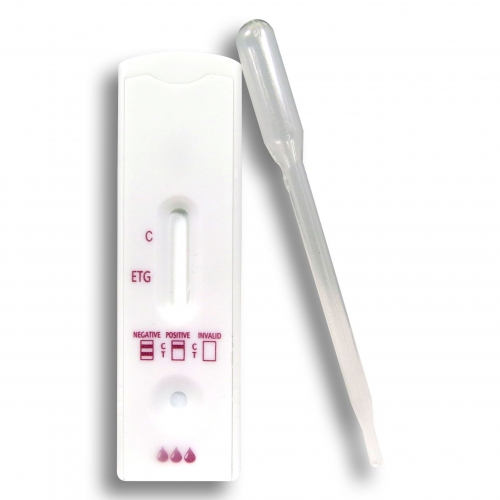
- Ethyl Glucoronide (EtG) Urine Alcohol Test: The EtG (Ethyl Glucoronide) urine Alcohol test checks for the presence of Ethyl Alcohol metabolite, named Ethyl Glucuronide. The detection period is generally up to 80 hours after ingestion. The EtG test is also known as “80 hour test” for detection of any amount of consumed Ethyl Alcohol within the last 80 hours. EtG has emerged as the preferred choice for Alcohol detection due to its presence for a longer period of time in contrast to Ethyl Alcohol. It can thus be used to detect recent alcohol consumption, even after Ethanol is no longer measurable. EtG is detectable in chronic drinkers for 80 hours or even up to 5 days. The factors that determine the detection window includes volume of Alcohol consumed and the time duration between each drink. Alcohol can be accurately detected by this test within 10 to 12 hours via standard methods, or 3 to 5 days when testing for EtG (Ethyl Gluconoride) metabolites. EtG urine tests are most commonly used to check individuals who are prohibited from drinking alcohol by the legal system or employers to determine whether they are consuming Alcohol within the past several days. However, urine testing may not be employed at specific times as in case of car accident.
-
Hair Alcohol Test (based on Ethyl Glucoronide (EtG) and Fatty Acid Ethyl Esters (FAEEs)):
It is a less common way for Alcohol detection via hair samples. Hair samples of length ranging from 3 cm to 6 cm are typically taken from a person’s scalp and analyzed for the presence of EtG (Ethyl Gluconoride) and FAEE (fatty acid ethyl esters), metabolites of Ethyl Alcohol that remain in the body following consumption of Alcohol.
Although it does not determine the present status of an individual under the influence of Alcohol, it can accurately reveal whether someone has consumed Alcohol within the past 90 days.
An alcohol hair test is commonly employed by legal officials and/or employers to individuals with a history of DUIs (driving under the influence) who are prohibited from drinking alcohol.

-
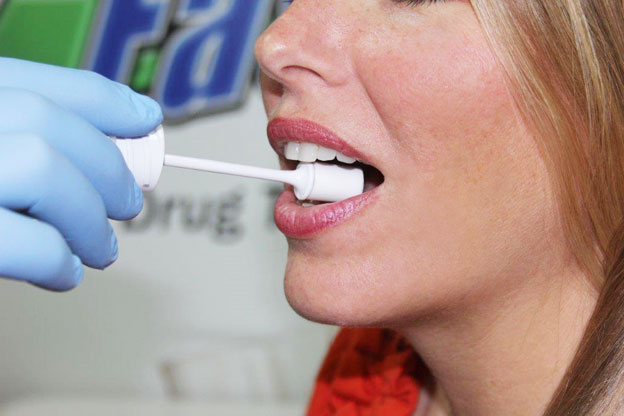
Saliva tests:
These tests are used to accurately determine the quantity of Alcohol within an individual’s saliva. Saliva testing usually performed for determining the presence of Alcohol can yield results within 2 to 5 minutes and can determine whether an individual has used Alcohol the past day.
The test involves collection of saliva mainly from the simple swab of the inner cheek and serves as an alternative to breath and blood test due to its ease to perform, less invasive nature and accurate results (the concentration of Alcohol in saliva is generally similar to the concentration of Alcohol within blood).
How to minimize the Blood Alcohol Concentrations?
The most effective way to maintain a low Blood-Alcohol Concentration (BAC) is to avoid consumption of large quantity of Alcohol (especially over a short-duration). The following are the different ways that can help to metabolize Alcohol and/or maintain a lower BAC.
- Avoid binge drinking: People should avoid binge drinking (consumption of huge amounts of Alcohol over a short duration of time with the intention of becoming intoxicated) to avoid the rise in BAC and thereby toxic burden on liver.
- Drink lots of water: The body should be kept sufficiently hydrated by consuming large amounts of water so that hydrated body can easily remove or filter out all toxic materials including Alcohol.
- Choose low alcohol-by-volume (ABV drinks): It is generally recommended to choose a drink with low ABV to stay under the legal driving limit of 0.08 BAC. A standard 12 oz beer generally contains lower amount of alcohol-by-volume (ABV) than compared to mixed drinks containing whiskey, gin, or vodka.
- Stay in shape: Individuals with high fat content and low muscle are not able to metabolize Alcohol very efficiently and result in higher BAC within a shorter duration from fewer drinks in comparison to those with low fat and high muscle.
- Non-alcoholic beverages: Consumption of more non-alcoholic beverages helps to avoid a high BAC and help the body to eliminate Alcohol quicker due to less consumption of Alcohol.
- Eat food: Presence of food in the stomach will slow the processing of Alcohol and help in attaining peak BAC within 30 minutes to 2 hours of drinking in contrast to consuming Alcohol on an empty stomach that results in more rapid processing and attainment of peak BAC between 1 and 6 hours of drinking.
- Sip slowly: Slow sipping helps the Alcohol to get slowly in the body and maintain a low BAC. To further enhance the benefits from slow sipping, it is recommended to increase the time intervals between sips, and decrease the amount of Alcohol per sip.
“How long does Nicotine stay in your System, Blood, Urine, Hair and Tests“
“Can you drink alcohol and take Aleve?”