Contents
- What is human growth hormone (hGH)?
- Synthetic forms of human growth hormone (hGH)
- Human growth hormone (hGH) structure
- Human growth hormone (hGH) release in the body
- Stimulators and inhibitors of human growth hormone seceretion (hGH)
- Human growth hormone (hGH) mechanism of action
- Human growth hormone (hGH) effects in the body
- Different products and forms of synthetic human growth hormone (hGH)
- Human growth hormone (hGH) pharmacokinetics
- Human growth hormone (hGH) uses
- Human growth hormone products for the treatment of growth failure in children
- Human growth hormone products for the treatment of Growth hormone deficiency in adults
- Human growth hormone (hGH) doses
- Human growth hormone (hGH) abuse in sports
- How is hGH most commonly abused in sports?
- Human growth hormone (hGH) doping effects
- Human growth hormone testing
- Human growth hormone side effects
- Human growth hormone toxicity mechanisms
- Can human growth hormone (hGH) therapy cause withdrawal effects
- Human growth hormone (hGH) use during pregnancy
- Human growth hormone (hGH) anti-aging effects
What is human growth hormone (hGH)?
Human growth hormone (hGH) or only growth hormone (GH), or somatotropin is a peptide-like hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals.
It is therefore very important for human development. It is a type of mitogen which is specific only to certain kinds of cells.
hGH contains 191-amino acid, single-chain polypeptide that is produced, stored and secreted by somatotropic cells localized at the lateral wings of the anterior pituitary gland.
It is also a stress hormone that raises the concentration of glucose and free fatty acids. It also stimulates production of IGF-1.
Synthetic forms of human growth hormone (hGH)
During the early 1980s, the development of recombinant growth hormone (rhGH) using recombinant technology resulted in the replacement of human pituitary extracts with rhGH for those children with growth hormone deficiencies.
The US Food and Drug Administration (FDA) approved the clinical use of rhGH (Protropin ®, Genentech, South San Francisco, CA) for the treatment of growth hormone deficiency in 1985.
In 1987, researchers elucidated the amino acid sequence of the growth hormone receptor, demonstrating a new class of transmembrane receptors.
Later that year, scienttists determined the 3 – dimensional crystalline structure of porcine GH, which is similar to hGH.
In 1996, the FDA approved the use of rhHG for the treatment of wasting or cachexia in patients with acquired immunodeficiency syndrome (AIDS).
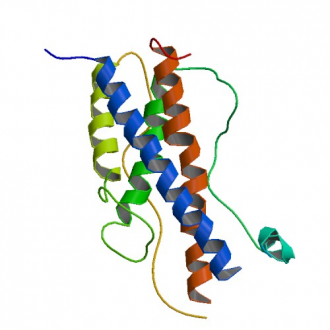
Human growth hormone (hGH) structure
About 70 – 75% of circulating hGH (Somatropin) is a 191 – residue, 22 kDa peptide, whereas about 5 – 10% occurs as a 20 kDa isoform as a result of alternative mRNA splicing.
Dimers and oligomers of hGH also occur in the plasma along with acidic, acylated, deaminated, and fragmented forms.
Recombinant hGH (rhGH) occurs only as a 22 kDa peptide. The binding of hGH to the 2 different growth hormone – binding proteins increases the heterogeneity of hGH isoforms in plasma.
The practical consequence of the wide variation in hGH isoforms is the substantial variation in the affinity of different immunoassays for the natural isoforms and fragments of hGH.
Recombinant human growth hormone occurs only in the 22 kDa – form that allows differentiation of exogenous hGH use by examining the ratio of 22 kDa – and non – 22 kDa – forms.
The 22 kDa GH polypeptide has the three-dimensional fold of a four-helical bundle protein, a configuration shared with other cytokines such as prolactin, erythropoietin, and many interleukins.
Crystal structure analyses indicate the first two helices are parallel to each other and antiparallel to the remaining two helices, with long connecting loops between the first two helices and second two helices.
Of the two disulfide bonds, one links the N-terminal and C-terminal regions (Cys53- Cys165). The second disulfide bond is located at the C-terminus.
Human GH also carries two zinc (Zn2+)-binding sites, identified at residues His44 and Glu200. The binding of Zn2+ is critical for facilitating GH dimerization and aggregation in the secretory granule biogenesis, which occurs in the anterior pituitary.
This is an important GH storage mechanism that permits rapid release of GH in response to appropriate stimulation without necessitating de novo protein synthesis
Human growth hormone (hGH) release in the body
Like many hormones, hGH is released episodically and is regulated by tightly controlled feedback pathways. This tightly controlled process provides a mechanism that has presumably been optimized biologically for hormone-receptor interactions resulting in activation, inactivation, and reactivation of signal transduction cascades.
The frequency of GH pulses appears to be conserved in all mammalian species, occurring at approx 3-h intervals. How this frequency is regulated is a topic that continues to hold great fascination for scientists.
Historically, hGH secretion was considered to be regulated by a positive/negative feedback loop controlled by the two hypothalamic hormones, GHRH and somatostatin.
GHRH is released from arcuate neurons into the median eminence and transported through the portal vessels to the pituitary gland, where it stimulates GH release from somatotrophs.
Negative feedback is thought to be mediated by GH stimulating the release of somatostatin from hypothalamic neurons. sst acts to inhibit GH release from the pituitary gland.
Stimulators and inhibitors of human growth hormone seceretion (hGH)
Stimulators of growth hormone (GH) secretion include:
- peptide hormones
- GHRH(somatocrinin) through binding to the growth hormone-releasing hormone receptor (GHRHR)
- ghrelin through binding to growth hormone secretagogue receptors (GHSR)
- sex hormones
- increased androgen secretion during puberty (in males from testis and in females from adrenal cortex)
- estrogen
- clonidineand L-DOPA by stimulating GHRH release
- α4β2 nicotinic agonists, including nicotine, which also act synergistically with clonidine.
- hypoglycemia, arginine and propranolol by inhibiting somatostatin release
- deep sleep
- niacinas nicotinic acid (Vitamin B3)
- fasting
- vigorous exercise
Inhibitors of GH secretion include:
- GHIH (somatostatin) from the periventricular nucleus
- circulating concentrations of GH and IGF-1(negative feedback on the pituitary and hypothalamus)
- hyperglycemia
- glucocorticoids
- dihydrotestosterone
Human growth hormone (hGH) mechanism of action
hGH acts in the body by binding to the human growth hormone receptor (GHR). After binding, hGH induces dimerization of GHR and activation of the GHR-associated JAK2 tyrosine kinase, and tyrosyl phosphorylation of both JAK2 and GHR.
These effects recruit and/or activate different signaling molecules, such as MAP kinases, phosphatidylinositol 3′ phosphate kinase, insulin receptor substrates, diacylglycerol, protein kinase C, intracellular calcium, and Stat transcription factors.
These signaling molecules lead to the GH-induced changes in gene expression, enzymatic activity, and transport function, that ultimately culminate in changes in growth and metabolism.
Human growth hormone (hGH) effects in the body
hGH exhibits its effects as an anabolic hormone in the body. Similarly to most other protein hormones, hGH acts by interacting with a specific receptor on the surface of cells.
Increased height during childhood is probably the most important effect of hGH. Additionally, hGH can:
- Increase calcium retention and strengthens and increases the mineralization of bone
- Increase muscle mass through sarcomere hypertrophy
- Promote lipolysis
- Increase protein synthesis
- Stimulate the growth of all internal organs excluding the brain
- Play a role in homeostasis
- Reduce liver uptake of glucose
- Promote gluconeogenesis in the liver
- Contribute to the maintenance and function of pancreatic islets
- Stimulate the immune system
- Increasesdeiodination of T4 to T3
Different products and forms of synthetic human growth hormone (hGH)
Human growth hormone is available in injectable forms derived from recombinant technology including Genotropin ® (Pfizer, New York, NY), Norditropin ® (Novo Nordisk, Inc., Princeton, NJ), Nutropin ® (Genentech, South San Francisco, CA), Humatrope ® (Eli Lily, Indianapolis, Indiana), Serostim ® (Serono, Inc., Rockland, MA), and Saizen ® (Serono, Inc., Rockland, MA). These commercial recombinant forms of hGH have the following 2 origins:
1) Modified Escherichia coli strain (Genotropin ®, Norditropin ®, Nutropin ®, Humatrope ® )
2) Mammalian cell line (mouse C127) (Serostim ®, Saizen ® ). The molecular weight of the rhGH is 22.125 kDa, identical to the 22 kDa – a form of endogenous hGH.
As a result of the popularity of hGH as an antiaging agent, all – natural supplements have been formulated as a low – cost method to stimulate the endogenous secretion of hGH.
These naturopathic preparations contain a variety of amino acids ( l – arginine, l – isoleucine, l – glutamine, l – glycine, l – lysine, l – tyrosine, l – valine) that are available as capsules, pills, powders, and nasal sprays. Somatrem ( N – methionyl – human growth hormone) was thefirstt biosynthetic form of growth hormone that contained an extra methionyl amino acid on the N – terminus (i.e., 192 amino acid residues).
The commercial form of somatrem was Protropin ® (Genentech), but manufacture of this form by Genentech ceased in 2004. Sustained release preparations of rhGH are now available for the treatment of growth hormone deficiencies that allow injections every 1 – 2 weeks instead of daily injections.
Human growth hormone (hGH) pharmacokinetics
Absorption. Absorption of hGH depends on several factors including the formulation, site of injection, and delivery method (SC, IM); prolonged absorption may result from depots of hGH in SC or IM tissue.
Following SC administration of rhGH, peak serum hGH concentrations occur within approximately 3–6 hours.
Typically, the bioavailability of subcutaneously injected hGH in high therapeutic doses ranges from approximately 50 – 70%; there are inadequate pharmacokinetic data to determine the bioavailability of supratherapeutic hGH doses used in sports doping.
Intranasal absorption of physiologic amounts of hGH requires the presence of a permeability enhancer.
Distribution
The estimated volume of distribution for hGH in healthy adults receiving therapeutic doses of hGH is < 1 L/kg; as a peptide, hGH probably distributes into the extracellular water.
Animal studies indicate that hGH can penetrate the blood-brain barrier and enter the central nervous system despite the absence of a specific transport system for hGH.
Metabolism and exretion
Biotransformation of hGH occurs primarily by metabolism in the liver, kidney, and peripheral tissues with only small amounts (i.e., < 0.01%) of hGH excreted unchanged by the kidneys based on rodent studies. The renal tubules reabsorb most of the hGH filtered by the glomeruli.
The clearance of hGH depends on several variables including hGH concentrations, body composition, insulin, growth hormone – binding protein, and IGF – binding protein – 1.
The effect of age on hGH clearance is minimal. The plasma elimination half – life of rhGH following IV infusion is very short (i.e., about 15 minutes).
The estimated mean plasma half – life of hGH in is about 17 minutes. Although the plasma half-life of hGH and rhGH is similar following IV administration, the terminal serum elimination half – life of rhGH following SC administration is 2 – 4 hours following single SC injections as a result of prolonged absorption and distribution.
Serum hGH concentrations are not usually detectable within 20 – 24 hours after SC injection of therapeutic doses. Recombinant hGH probably does not degrade into the 20 – kDa isoform.
Human growth hormone (hGH) uses
hGH is used in the treatment of dwarfism and growth failure, as it stimulates skeletal growth in children with growth failure due to a lack of adequate secretion of endogenous GH.
Skeletal growth is accomplished at the epiphyseal plates at the ends of a growing bone. Growth and metabolism of epiphyseal plate cells are directly stimulated by GH and one of its mediators, IGF-I (insulin-like growth factor).
FDA approved indications for hGH are:
- Adult onset growth hormone deficiency
- Cachexia
- Growth Failure
- Short Bowel Syndrome (SBS)
- Short Stature
- Childhood onset growth hormone deficiency
Since the early 1990s, growth hormone has also been promoted as an antiaging drug. During this time, human studies suggested that growth hormone – deficiencies in elderly patients accounted for reduction in lean body mass, increases in fat mass, and thinning of the skin.
However, currently there is no clear evidence that the use of hGH improves longevity or the quality of life.
Human growth hormone products for the treatment of growth failure in children
Different hGH products can be used for the treatment of growth failure in chi the dren in following situations:
- Treatment of growth failure due to inadequate endogenous growth hormone secretion (Genotropin, Humatrope, Norditropin, Nutropin, Nutropin AQ, Omnitrope, Saizen, Tev-Tropin, Zomacton).
- Treatment of short stature associated with Turner syndrome (Genotropin, Humatrope, Norditropin, Nutropin, Nutropin AQ, Omnitrope).
- Treatment of Prader-Willi syndrome (Genotropin, Omnitrope).
- Treatment of growth failure associated with chronic kidney disease up until the time of renal transplantation (Nutropin, Nutropin AQ).
- Treatment of growth failure in children born small for gestational age who fail to manifest catch-up growth by 2 years of age (Genotropin, Omnitrope) or by 2 to 4 years of age (Humatrope, Norditropin)
- Treatment of idiopathic short stature (nongrowth hormone-deficient short stature), defined by height standard deviation score (SDS) ≤-2.25 and growth rate not likely to attain adult height in the normal range, in pediatric patients whose epiphyses are not closed and for whom other causes associated with short stature have been excluded (Genotropin, Humatrope, Nutropin, Nutropin AQ, Omnitrope).
- Treatment of short stature or growth failure associated with short stature homeobox gene (SHOX) deficiency (Humatrope).
- Treatment of short stature associated with Noonan syndrome (Norditropin)
Human growth hormone products for the treatment of Growth hormone deficiency in adults
Replacement of endogenous growth hormone in adults with growth hormone deficiency who meet either of the following criteria (Genotropin, Humatrope, Norditropin, Nutropin, Nutropin AQ, Omnitrope, Saizen):
- Adult-onset: Patients who have adult growth hormone deficiency whether alone or with multiple hormone deficiencies (hypopituitarism) as a result of pituitary disease, hypothalamic disease, surgery, radiation therapy, or trauma
- Childhood-onset: Patients who were growth hormone deficient during childhood as a result of congenital, genetic, acquired, or idiopathic causes, confirmed as an adult before replacement therapy is initiated
Human growth hormone (hGH) doses
The recommended dose of hGH depends on the medical condition, gender, and age, titrated to response (e.g., normalization of plasma IFI – I concentrations in adults) and the presence of side effects.
In children with growth hormone deficiency, the usual daily doses range from 5 – 25 μg/kg body weight. In general, the daily dose of rhGH in GH – deficient adults is smaller than doses in children, typically beginning as 150 – 300 μg and titrated up to 1 mg (3 IU). High – dose regimens in growth hormone – deficient children use up to 80 μg/kg.
Although the use of SC rhGH is approved for use in adult cachexia, there is no clear benefit from the use of rhGH in these patients.
Off – label uses of rhGH include the treatment of obesity, osteoporosis (70 μg/kg/week divided in 3 subcutaneous injections for 12 months), muscular dystrophy, and infertility, but there are inadequate clinical data to support of effi cacy and safety of rhGH for these uses.
Human growth hormone (hGH) abuse in sports
The use of hGH in athletics for the enhancement of size, athletic performance, and muscle strength began in the 1980s, particularly among swimmers, cyclists, and competitive bodybuilders.
Although this substance was initially promoted for strength disciplines (e.g., football, baseball), the lipolytic actions of hGH was attractive to endurance athletes as a means to reduce fat mass.
This hormone was touted as an expensive, fashionable new athletic drug by the steroid guru, Dan Duchaine. Growth hormone was promoted as an anabolic agent that had synergistic effects with anabolic steroids.
Ben Johnson, the former Canadian sprinter and world record holder in the 100 meter dash, admitted using a regimen including hGH after his gold medal was revoked following a positive steroid test at the 1988 Seoul Olympic Games.
These regimens gained popularity because of the difficulty detecting the use of hGH. In 1996, the International Olympic Committee launched the Human Growth Hormone 2000 project with the goal of developing reliable screening tests for the use of hGH, but the search for sensitive tests for hGH remains complicated.
Recombinant human growth hormone (rhGH) was confiscated at a variety of elite athletic events including the 1998 World Swimming Championships and the Tour de France.
The 1994 World Aquatic Championship breast stroke silver medalist, Yuan Yuan was sent home from the 1998 World Aquatic Championships in Perth, Australia when she was caught trying to smuggle 13 vials of hCG into Australia.
As a result of the difficulty detecting rhGH use more than a day after cessation of rhGH use, the first athlete disciplined for rhGH use occurred in 2010.
A UK rugby player, Terry Newton tested positive for hGH in a blood sample, and he received a 2 – year ban for using rhGH ostensibly to increase muscle mass.
How is hGH most commonly abused in sports?
The administration of hGH usually involves the subcutaneous (SC) or intramuscular (IM) injection of pharmaceuticp reparations diverted from legal sources. Typically, the use of hGH is part of a regimen that includes anabolic steroids.
Human growth hormone (hGH) doping effects
Effects on bone and growth
Promotion of longitudinal growth clearly is the main effect of rhGH in children, but it also has effects on bone in later life.
One might speculate that increasing height in children or adolescents could be advantageous if a career is planned in disciplines like basketball or high jumping.
Although no numbers are available on the frequency of doping in children and adolescents, this issue is obviously of concern.
Effects on metabolism and body composition
Effects of hGH on substrate metabolism have been known for many years. In particular, the fact that hGH is a potent lipolytic agent makes it very interesting for the cheating athlete.
Hyposomatropism reduces lipolysis in abdominal obesity. Potentially increasing muscle mass at the cost of a decrease in fat mass is definitely what many athletes want to achieve.
However, from a scientific point of view it is important to keep in mind that most data were obtained from studies in hGH-deficient patients.
For example, it is well documented that in hGH-deficient adults recombinant hGH replacement therapy leads to profound changes in body composition, the effect being more pronounced in males than in females.
In trained healthy subjects only a few controlled studies have been performed on this topic, with much less pronounced results: one study using comparatively high doses showed a decrease in body fat and an increased fat-free body weight.
Furthermore, dose-dependent changes in body composition were observed in a double-blind placebo-controlled study in subjects without GHD with increased muscle mass.
The small increase in fat-free mass in the GH-treated groups in another study was explained as a consequence of fluid retention or accumulation of connective tissue rather than accretion of contractile muscle protein.
A placebo-controlled 6-week trial in power athletes did not demonstrate a significant effect on body composition. 12-week double-blind placebo-controlled study in healthy women was conducted, showing a marked increase in energy expenditure and fat combustion.
However, the participants in this study were elderly women, and it is difficult to extrapolate those finding to the situation in highly trained athletes.
In conclusion, the well established lipolytic effect of recombinant hGH administration is most likely dependent on the individual body composition before treatment and attenuated in healthy trained adults in comparison to GHD patients.
Effects on protein turnover, muscle mass and strength
Growth hormone is known to stimulate protein turnover, and in some studies it is considered a strong anabolic agent. This might be the most important reason for its popularity in sports.
Again, starting with lessons learned from treatment of hGH deficient patients, it is obvious that hGH has an effect on muscle mass.
Reduced skeletal muscle mass and muscle strength are found in GHD adult patients and are reversible by replacement therapy with hGH. On the other hand, a chronic excess of hGH such as in acromegaly leads to voluminous, but functionally not improved skeletal muscles.
In an animal model of GH transgenic mice, growth hormone was less effective in increasing muscle weight than body weight, and muscle strength did not increase proportionally with muscle weight.
Comparing muscle contraction capacity in childhood-onset GHD patients to that of normal controls raised doubts that weakness and fatigability in GHD patients originate from the skeletal muscle itself.
In obese and normal-weight healthy women, it has been shown that hGH administration can block protein oxidation and stimulate protein turnover.
However, beneficial effects of supraphysiological hGH administration on muscle strength or function have not yet been demonstrated in trained athletes.
In a meta-analysis of studies on hGH and muscle strength, it was concluded that overall there is no evidence of increased muscle strength with hGH in healthy trained subjects and it has been questioned if there are anabolic effects in healthy subjects at all.
Effects on cardiac and pulmonary function
Many childhood-onset GHD patients have an impaired lung volume. A decreased maximal expiratory pressure was found in both childhood-onset and adult-onset GHD patients.
Treatment with rhGH improves ventilatory function, oxygen uptake (VO2max) and exercise capacity in GHD adults. GH treatment is also used children with cystic fibrosis to improve exercise tolerance, most likely through effects on muscular, cardiovascular and pulmonary capacity.
However, a placebo-controlled study in intensive care unit patients requiring mechanical ventilation did not show beneficial effects of rhGH treatment on respiratory function.
If GHD patients are treated with GH, cardiac muscle mass and maximal cardiac output increase, but if elevated hGH levels in acromegalic patients are reduced, cardiac function is also improved.
As for many pharmacological GH effects, and also for the effects on heart or lung capacity, data are scarce in healthy trained subjects.
Human growth hormone testing
The detection of exogenous hGH is complicated by the presence of endogenous hGH. Radioimmunoassay (RIA) and immunoradiometric assays (IRMA) detect whole hGH isoforms, whereas immunofunctional assay methods (IFA) detect only biologically active hGH isoforms.
Consequently, the hGH concentrations by IFA are about 27 – 30% lower than determinations by RIA or IRMA because of the interference by GH – binding protein and the inclusion of several different molecular forms of hGH by the latter methods. IFA is an ELISA method that incorporates 2 binding sites of the transmembrane growth hormone receptor.
Nonfunctional isoforms do not bind to the growth hormone – binding protein; therefore, these isoforms of hGH are not detected by this method.
However, the presence of isoform fragments, which contain binding sites for antibodies, can cause overestimation of the serum hGH concentration when high concentrations of rhGH are present.
Human growth hormone side effects
Adverse effects of hGH during medical use include insulin resistance, pre- and postpubertal gynecomastia, arthralgias, edema, headache, erectile dysfunction, low-grade fevers, and carpal tunnel syndrome.
In clinical trials using hCG to treat GH – deficient adult patients, the most common side effects are edema, arthralgias, and myalgias.
The large muscle mass associated with acromegaly is associated with myopathy and relative weakness. Case reports associate sudden death with the early treatment phase of Prader-Willi syndrome with growth hormone, but the presence of other potential complicating factors (e.g., pneumonia, sleep apnea, pulmonary hypertension) limit conclusions regarding the causal role of hGH.
Complications of treating familial short stature with rhGH include nonketotic hyperglycemia, hypothyroidism, benign intracranial hypertension, and acute pancreatitis.
In patients with GH deficiency, replacement therapy with rhGH improves mood and sense of well-being.
Clinical studies indicate that withdrawal of rhGH treatment from adults with severe GH deficiency produces detrimental psychologic effects manifest by fatigue, pain, irritability, and depression.
Rarely, case reports associated the development of hypersensitivity reactions (pruritus, urticaria) with rhGH therapy, particularly with the use of somatrem.
Human growth hormone toxicity mechanisms
Human growth hormone is the main stimulus for the release of IGF-1. The effects of hGH are primarily mediated by the action of IGF – 1; consequently, the adverse effects of hGH and IFG – I are expected to be similar to diseases associated with growth hormone excess (i.e., acromegaly with fluid retention, hypertension, cardiomyopathy, and diabetes mellitus).
Normal hGH levels are an important factor in the development and function of the cardiovascular system.
Although hGH improves muscle and cardiac function in hGH – deficient patients, there are few data on the effects of chronic hGH use by healthy subjects on the risk of increased morbidity and/or mortality from cardiovascular disease.
Chronic hGH decreases high – density lipoprotein (HDL), which is a risk factor for coronary heart disease. Chronic growth hormone defi ciency is associated with impaired cardiac performance, reduced left ventricular systolic function, dilated cardiomyopathy, and congestive heart failure.
Growth hormone excess (acromegaly) is associated with an increased risk of hypertension, cardiomyopathy, cardiovascular disease, diabetes mellitus, osteoporosis, and impotence.
Can human growth hormone (hGH) therapy cause withdrawal effects
An abstinence syndrome associated with cessation of the chronic use of rhGH in healthy subjects is not well defined.
Physiologically, the administration of exogenous hGH suppresses the secretion of endogenous hGH, but there are few data on the clinical and pathologic effects of chronic suppression of hGH secretion.
In patients with severe growth hormone deficiencies, withdrawal of rhGH therapy results in fatigue, pain, irritability, and depression.
However, the effects of hormone deficiency are difficult to separate from a true withdrawal syndrome.
Human growth hormone (hGH) use during pregnancy
There are no well-controlled studies of reproductive abnormalities associated with the use of rhGH in healthy subjects. The FDA classifi es rhGH in pregnancy category B (fetal harm possible, but unlikely) and somatrem in pregnancy category C (lack of animal and human studies).
Human growth hormone (hGH) anti-aging effects
Except on growth, hGH has also effects on body composition. People with a significant hGH deficiency, usually due to pituitary disease, have increased body fat and decreased muscle mass and bone density.
Such changes in hGH-deficient patients mimic aging. Indeed, one study found that a small number of older men who were given hGH had improved muscle mass, decreased body fat, and better bone density. There have since been numerous claims that hGH is the “anti-aging miracle.”
One large meta-analysis on hGH effects showed that long-term use of hGH caused an average 2.3-kilogram loss of weight, 2.6 kg loss of fat, 1.4 kg (3 lbs) increase in lean body mass, and no consistent change in bone density. Patients feel generally better, as seen in the quality of life scores.
However, the effect on longevity is not yet known. There is a paradox that both hGH deficiency and hGH excess, in a disease called acromegaly, are associated with shorter life expectancy. Further, even though there is an increase in mortality in pituitary patients missing HGH, there is no evidence that this it improved with hGH treatment.