Contents
- What is lansoprazole?
- Lansoprazole chemistry and drug class
- What is the mechanism of action of lansoprazole?
- What is the pharmacokinetics of the Lansoprazole?
- How long lansoprazole stays in your body?
- What are the uses of Lansoprazole?
- What are the precautions one should take while using Lansoprazole?
- Who cannot take lansoprazole?
- What are the contraindications of the lansoprazole?
- What are the side effects of lansoprazole?
- What is the dosage of lansoprazole?
- What should I do in case of the missed dose of lansoprazole?
- What should I do in case of the overdose of lansoprazole?
- Can I take lansoprazole in pregnancy?
- Can I take lansoprazole while breastfeeding?
- What are some drugs that can interact with lansoprazole?
- Can lansoprazole be taken with delavirdine?
- Can lansoprazole be taken with fluvoxamine?
- Can lansoprazole be taken with sucralfate?
- Can lansoprazole be taken with aminophylline?
- Can lansoprazole be taken with ketoconazole?
- Can lansoprazole interact with laboratory markers?
- Can lansoprazole be taken with aspirin?
- Can lansoprazole be taken with atorvastatin?
- Can lansoprazole be taken with cyanocobalamin?
- Can lansoprazole be taken with bisacodyl?
- Can lansoprazole be taken with methotrexate?
- Can lansoprazole be taken with amoxicillin?
- Can lansoprazole be taken by a patient having liver diseases?
- Can lansoprazole be given in conditions like bone fractures?
- Can lansoprazole cause acute interstitial nephritis?
- Can lansoprazole provoke Clostridium difficile-associated diarrhea
- Lansoprazole use and cutaneous or systemic lupus erythematosus
- Can lansoprazole give false positive results in the diagnosis of neuroendocrine tumors?
- Can lansoprazole be given in hypomagnesemia or magnesium imbalance?
- Can lansoprazole be used in pediatrics patients?
What is lansoprazole?
Lansoprazole is a proton pump inhibitor which prevents the stomach from producing acid. Lansoprazole has been marketed for many years and is one of several PPI’s available.
It is used to treat and prevent stomach and intestinal ulcers, erosive esophagitis (damaged esophagus due to stomach acid) and other conditions involving excessive stomach acid such as Zollinger-Ellison syndrome.
It is also known to treat frequent heartburn that happens 2 or more days per week and is available over the counter for such use. However, it is not beneficial in providing the instant relief from heartburn symptoms.
Lansoprazole chemistry and drug class
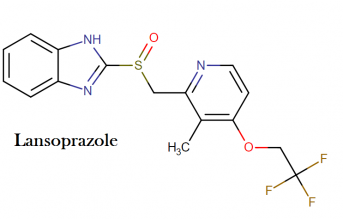
Molecular structure:
Molecular formula: C16H14F3N3O2S
Molecular weight: 369.362 g/mol
IUPAC name: 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole
Drug class: This compound belongs to the class of organic compounds known as sulfinylbenzimidazoles. These are polycyclic aromatic compounds containing a sulfinyl group attached at the position 2 of a benzimidazole moiety.
What is the mechanism of action of lansoprazole?
Lansoprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or histamine H2-receptor antagonist properties, but rather suppress gastric acid secretion by specific inhibition of the (H+, K+)-ATPase enzyme system at the secretory surface of the gastric parietal cell.
Because this enzyme system is regarded as the acid (proton) pump within the parietal cell, lansoprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated gastric acid secretion irrespective of the stimulus.
What is the pharmacokinetics of the Lansoprazole?
After oral administration, theCmax occurss in 1.7 hours as the absorption is rapid and complete with an absolute bioavailability of over 80%.It shows 97% of protein binding.
The metabolism of lansoprazole is hepatic. Hydroxylated sulfinyl and sulfone derivatives of lansoprazole are two metabolites of lansoprazole which are identified in measurable quantities in plasma.
These metabolites have very little or no antisecretory activity. Lansoprazole is metabolized in two active species which inhibit acid secretion by ATPase enzyme within parietal cell canaliculus.
How long lansoprazole stays in your body?
The elimination half-life of lansoprazole is 1 to 2 hours, therefore, it will be out of your system in one day completely.
It is known that despite having a shorter elimination half-life lansoprazole provide effective gastric acid suppression over 24 hours and a prolonged pharmacological action. In repeated dosing of 7 days, a patient can achieve around 90% inhibition of stimulated acid secretion.
What are the uses of Lansoprazole?
Lansoprazole is beneficial in treating diseases like:
- Gastroesophageal Reflux (GERD): Lansoprazole is effective as a short-term treatment of gastroesophageal disease with symptoms such as heartburn, erosive esophagitis which is diagnosed endoscopically in patients with GERD. It effectively maintains the healing and reduces the recurrences of erosive esophagitis. Lansoprazole acts as a short-term self–medication for symptomatic relief of frequent (such as – more than twice per week) heartburn in adults ≥18 years of age.
- Duodenal ulcer: Lansoprazole is a short-term treatment of active duodenal ulcers which are endoscopically or radiographically confirmed. It is also used to treat Helicobacter pylori infection and duodenal ulcer disease. It has been used for multidrug regimens as triple therapy and dual therapy, for example, clarithromycin and clarithromycin + amoxicillin. In order to maintain duodenal ulcer healing, it is used as maintenance therapy.
- Gastric ulcer: Short-term treatment and symptomatic relief of active benign gastric ulcer.
- NSAIA-induced gastric ulcer: Prolonged usage of nonsteroidal anti-inflammatory agents can induce gastric ulcers and lansoprazole is effective as a short-term treatment of such gastric ulcers. It effectively reduces the risk in a patient with a history of gastric ulcers who may require NSAIA treatment.
- Pathologic GI hypersecretory conditions: In severe conditions such as Zollinger-Ellison syndrome which is with or without multiple endocrine adenomas, lansoprazole can be used as a long-term treatment of pathologic hypersecretory conditions.
- Crohn’s disease-associated ulcers: Some evidence for use of proton-pump inhibitors (e.g., omeprazole) for gastric acid suppressive therapy as an adjunct in the management of upper GI Crohn’s disease, including esophageal, gastroduodenal, and jejunoileal disease.
What are the precautions one should take while using Lansoprazole?
- The risk of Clostridium difficile infection can be increased by 2.75 times due to proton pump inhibitors such as lansoprazole. It also increases the risk of antibiotic-associated diarrhea, colitis or pseudomonas colitis. Even many other patients had a risk factor for colectomy and CDAD (C.difficile associated diarrhea). One should use the minimum required, lowest effective and shortest duration of therapy appropriate for patient’s clinical condition. Response to lansoprazole therapy does not preclude the presence of occult gastric neoplasm and can result in gastric malignancy.
- Lansoprazole can also cause phenylketonuria because the orally disintegrating tablet of lansoprazole contains aspartame, which is metabolized in the gastrointestinal tract. After disintegration, it provides 2.5 or 5 mg of phenylalanine that leads to phenylketonuria.
- The risk of many other infections such as respiratory tract infections (mainly community-acquired pneumonia) can be increased by administration of proton pump inhibitors.
- Increased risk of osteoporosis-related fractures of hip, wrist, or spine have been observed in many studies by the use of use of proton-pump inhibitors, particularly in high dosages (i.e., multiple daily doses) and/or for prolonged periods of time (i.e., ≥1 year). The magnitude of risk is unclear. The safety concern is being evaluated continuously by FDA. Depending upon patient’s condition, one should use lowest effective dosage and shortest duration of therapy.
- Patients, who are risk of osteoporosis-related fractures, should take adequate amount of calcium and vitamin D. One should monitor the bone health of these patients and is advisable to treat accordingly.
- In patients receiving long-term therapy with proton pump inhibitors including lansoprazole, symptomatic or asymptomatic hypomagnesemia. Serious adverse effects include tetany, seizures, tremors, carpopedal spasm, arrhythmias (e.g., atrial fibrillation, supraventricular tachycardia), and abnormal QT interval. Paresthesia, muscle weakness, muscle cramps, lethargy, fatigue, and unsteadiness may occur. Discontinuance and magnesium replacement is advisable for most patients and hypomagnesemia resolved within 1 week after discontinuation. However, It can recur again within 2 weeks of rechallenge.
- Patients currently receiving digoxin or drugs that may cause hypomagnesemia such as diuretics should measure serum magnesium concentration if they are expected to receive proton pump inhibitor therapy. The measuring of magnesium levels should be done prior to starting of PPIs therapy. It is advisable to have a control and monitoring of magnesium serum level thereafter also.
Who cannot take lansoprazole?
Lansoprazole is a prescription medication and it can be taken by adults and children as well when prescribed by a doctor. Lansoprazole in capsule formulation contains little amount of lactose which can be responsible for the digestive problems such as lactose tolerance, so if any person having a problem with the digestive system, they should avoid taking this medication.
Before taking this medication, let your doctor know if you have:
- Allergic reaction to any medication or lansoprazole itself in the past
- Liver problems
It is important to note that lansoprazole should not be taken by a pregnant woman until it is very necessary. (The pregnancy and breastfeeding warnings of the lansoprazole are given in this article).
What are the contraindications of the lansoprazole?
Lansoprazole is contraindicated in the patients with known severe hypersensitivity to lansoprazole or any ingredient in the formulation.
What are the side effects of lansoprazole?
If you have signs of an allergic reaction to lansoprazole such as hives, difficulty breathing, swelling of your face, lips, tongue, or throat, seeks medical help immediately.
Call your doctor at once if you have:
- Severe stomach pain, diarrhea that is watery or bloody
- New or unusual pain in your wrist, back, hip, or thigh
- A seizure (convulsions)
- Irregular heartbeats
- Kidney problems- little or no urination, blood in your urine, swelling, rapid weight gain
- Lupus-like syndrome- joint pain or swelling with fever, swollen glands, and muscle aches, chest pain, vomiting, unusual thoughts or behavior, and patchy skin color.
Common lansoprazole side effects may include:
- Nausea, stomach pain
- Diarrhea, constipation
- A headache
This is not a complete list of side effects and others may occur. Therefore as soon as you any unusual symptom of lansoprazole, call your doctor immediately.
What is the dosage of lansoprazole?
- Lansoprazole is marketed under the brand names Prevacid, Prevacid 24hour, and Prevacid Solutab.
- Lansoprazole is also available under the name First-Lansoprazole.
- Lansoprazole is available in capsule formulation as Prevacid capsules, which are available in 15 and 30 milligrams (mg). It is also available in delayed release formulation in 15 mg and 30 mg which are marketed as Prevacid 24 hour and Prevacid Solutab.
- Lansoprazole solutab when put beneath the tongue, it dissolves there.
- Lansoprazole 24 hour which is marketed as Prevacid 24 hour should be taken after every 24 hours or once a day for 14 days in a row. The complete effect can be seen in four days.
- It is very important to take this medication once a day, do not repeat the dose without prior consultation with a doctor.
- It is also available as over the counter medication and if you are taking as an OTC medication, stop using it after 14 days and if you don’t see any improvement in your condition, consult your doctor.
- A 14 days course of lansoprazole should not be repeated before 4 months.
- Call your doctor if you have additional symptoms and need treatment before the four months have passed.
- Before giving this medication to a child, consult a doctor. Child below the age of 1 year should not be given this medication.
What should I do in case of the missed dose of lansoprazole?
In case of the missed dose, take the missed dose as soon as you remember it, avoid taking the dose in case, you remember it near next scheduled dose. Do not double up this dose, if you are taking the missed dose near the next scheduled dose.
Go ahead and take your missed medication unless you are near the time for your next scheduled dose.
What should I do in case of the overdose of lansoprazole?
In case of the overdose of Lansoprazole, a patient can suffer the symptoms such as difficulty breathing, breaking out in hives, and swelling of the face, lips, tongue, or mouth.
Seek emergency medical help if you experience an overdose reaction from lansoprazole.
Can I take lansoprazole in pregnancy?
With high doses of PPIs, fetal mortality and lower fetal weight have been noticed in animal models. However, any teratogenic study has not been observed.
In human pregnancy, there is no controlled data is given. As per AU-TGA, it comes under pregnancy B3 category, as per this category, only a limited number of women of childbearing age, or pregnant woman, take this medication and not observed any kind of malformations or other harmful effects to the fetus.
The significance of increased occurrence of fetal damage in animal studies which is considered uncertain in humans.
As per USFDA, it comes under pregnancy category B: in which any animal studies failed to demonstrate any risk to the fetus, moreover, there are no adequate studies are given in human pregnancy. Therefore, the use of this medication is not advisable unless it is clearly needed.
Can I take lansoprazole while breastfeeding?
The use of lansoprazole during breastfeeding is not recommended. You should either discontinue the drug or discontinue the breastfeeding, even it is important for mother.
This medication is unknown to be excreted in the human milk, at the same time, effect of this medication on the nursing infant is unknown.
What are some drugs that can interact with lansoprazole?
Many drugs can interact with lansoprazole and you should always consult your doctor before taking this medication if are advised to take lansoprazole.
- Aminophylline
- Ampicillin
- Bisacodyl
- Clopidogrel
- Delavirdine
- Fluvoxamine
- Iron Salts
- Sucralfate
- Theophylline
- Astemizole
- Voriconazole
- Ketoconazole
This is not a complete list; always talk to your pharmacist about the safety of this medication.
Can lansoprazole be taken with delavirdine?
Proton pump inhibitor such as lansoprazole increases the gastric pH, which can lead to least absorption of delavirdine.
When taken together, this medication can cause a major interaction and the interaction cannot be eliminated by even separating the doses because these both drugs increase the gastric pH for an extended period of time. Chronic use of both of these medications is not recommended.
Can lansoprazole be taken with fluvoxamine?
When lansoprazole is taken with fluvoxamine, it causes moderate interaction. When administered concurrently, fluvoxamine can reduce the metabolism and increase the plasma concentration of the lansoprazole.
One should monitor the patient for the PPI toxicity like a headache or GI distress if these drugs are combined.
Can lansoprazole be taken with sucralfate?
The bioavailability and absorption of lansoprazole can be reduced by 17% when administered along with Sucralfate. If this is necessary to take both medications together than take lansoprazole at least 30 minutes before taking the sucralfate.
Can lansoprazole be taken with aminophylline?
Lansoprazole and theophylline administration cause a minor interaction. Concomitant use of theophylline, a CYP1A2 and CYP3A substrate, and lansoprazole has led to a small increase in theophylline clearance.
Aminophylline may require dosage adjustment when therapy with lansoprazole is initiated or discontinued. (Minor) Concomitant use of theophylline, a CYP1A2 and CYP3A substrate, and lansoprazole has led to a small increase in theophylline clearance. Theophylline may require dosage adjustment when therapy with lansoprazole is initiated or discontinued.
Lansoprazole is also known to reduce the area under the plasma concentration-time curve of theophylline. This effect appears to be small (approximately a 13% decrease in one study) and may be related either to a reduction in absorption or to enhanced clearance caused by induction of hepatic enzymes by lansoprazole.
Dosage adjustment is not believed to be necessary. However, close observation for evidence of altered methylxanthine effect is recommended.
Can lansoprazole be taken with ketoconazole?
One should generally avoid the administration of lansoprazole with ketoconazole. The ketoconazole or itraconazole require an acidic environment for dissolution and gastrointestinal absorption of these azole antifungal agents can be reduced by lansoprazole.
By reducing the acidity and increasing gastric pH, the lansoprazole scan decrease bioavailability of the azoles (ketoconazole) by 75% to 80%.
If the co-administration of both the medication is necessary than 15 minutes before taking the ketoconazole, an acidic pH must be produced with two capsules of glutamic acid hydrochloride. The aerated drink such as Coca-cola or Pepsi can also help in this regard.
However, at the same time, the desired antifungal effect of azoles can still not be expected. Other agents can be considered such as fluconazole or terbinafine whose absorption is not affected by the stomach pH.
Can lansoprazole interact with laboratory markers?
Yes, it can. Changes in laboratory parameters in patients who received lansoprazole were reported as adverse reactions:
- abnormal liver function tests
- increased SGOT (AST)
- increased SGPT (ALT)
- increased creatinine
- increased alkaline phosphatase
- increased globulins
- increased GGTP
- increased/decreased/abnormal WBC, abnormal AG ratio, abnormal RBC
- bilirubinemia
- blood potassium increased
- blood urea increased, crystal urine present
- eosinophilia
- hemoglobin decreased
- hyperlipemia
- increased/decreased electrolytes
- increased/decreased cholesterol, increased LDH,
- increased glucocorticoids
- increased/decreased/abnormal platelets
- increased gastrin levels and positive fecal occult blood
- Urine abnormalities such as albuminuria, glycosuria, and hematuria were also reported.
Can lansoprazole be taken with aspirin?
The oral bioavailability of aspirin and other salicylates can be seen when administered concurrently with proton pump inhibitors such as lansoprazole. The interaction has been studied with omeprazole and aspirin, although data are conflicting.
In one study, after administration of aspirin in 650 mg dose, the omeprazole have been observed to cause a significant and progressively greater reduction in the mean serum salicylates level in some healthy volunteers.
Here the scientists concluded that acid suppression can decrease the lipophilic nature of aspirin. And also affects its absorption on gastric tract.
In rats, omeprazole is observed to interfere with the analgesic, antipyretic, or anti-inflammatory effects of aspirin. In one another study, no effect of omeprazole pretreatment (20 mg/day for 4 days) on plasma salicylate and aspirin levels, skin bleeding times, or antiplatelet effect of low-dose aspirin (125 mg single dose) have been found.
Proton pump inhibitors may enhance the release rate of salicylates from enteric-coated formulations due to premature disruption of the coating and intragastric release of the drug secondary to an increase in gastric pH.
Can lansoprazole be taken with atorvastatin?
The plasma concentration of atorvastatin and associated risk of myopathy can be increased when both proton pump inhibitors (esomeprazole and lansoprazole) and atorvastatin are administered together.
This happens due to competitive inhibition of P-glycoprotein which results in reduced drug secretion into the intestinal lumen and enhanced drug bioavailability.
Another mechanism which can be considered as CYP4503A4 enzyme inhibition. The interaction was reported for in a patient who was taking atorvastatin (from more than one year) and take esomeprazole (for 6 weeks), and 2 days after addition of clarithromycin, he developed rhabdomyolysis along with AV block.
Severe symptoms such as increased fatigue, mild chest pain, and shortness of breath that coincided with the initiation of esomeprazole approximately six weeks prior to admission by the patient.
Theoretically, the interaction may also occur with other proton pump inhibitors like lansoprazole, omeprazole, and pantoprazole and HMG-CoA reductase inhibitors like lovastatin and simvastatin, since these drugs are all substrates of P-glycoprotein and CYP450 3A4.
The patients treated with atorvastatin, lovastatin, simvastatin, and red yeast rice (which contains lovastatin) should be monitored closely while using proton pump inhibitors because of increased risk of musculoskeletal toxicity associated with high levels of HMG-CoA reductase inhibitory activity in plasma.
The patient is advised to their doctor immediately if they experience any unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever, especially when they are on the treatment of HMG-CoA reductase inhibitors.
If myopathy is suspected or diagnosed, creatinine kinase is markedly elevated, therapy should be discontinued.
Can lansoprazole be taken with cyanocobalamin?
By reducing or suppressing gastric acid secretion, H2-receptor antagonists and proton pump inhibitors may interfere with the gastrointestinal absorption of vitamin B12, a process that is dependent on the presence of gastric acid and pepsin.
Clinical studies have shown that dietary (i.e., protein-bound) vitamin B12 malabsorption can occur during treatment with these agents, particularly proton pump inhibitors, although the likelihood of developing clinically significant deficiency over time is unknown.
There has been one reported case of vitamin B12 deficiency with megaloblastic anemia in a patient who received omeprazole at a minimum of 40 mg/day for 4 years.
Also uncertain is whether acid reduction or suppression can affect the absorption of vitamin B12 ingested in the form of oral supplements such as cyanocobalamin.
Non-oral routes of administration (e.g., parenteral, intranasal, sublingual) are generally preferred in the treatment of B12 deficiency-related anemia.
Can lansoprazole be taken with bisacodyl?
The gastric pH can be raised by the concomitant administration of bisacodyl oral tablets and other proton pump inhibitors.
The PPIs can prematurely dissolve the enteric coating of the bisacodyl which can cause gastric irritation or dyspepsia. One should avoid taking the PPIs immediately after or before bisacodyl. One should maintain a time gap of at least 1 hour in between both the medications.
Can lansoprazole be taken with methotrexate?
Studies suggest that concomitant use of methotrexate (primarily at high dose) and PPIs such as lansoprazole may elevate and prolong serum levels of methotrexate and/or its metabolite, leading to methotrexate toxicities.
In high-dose methotrexate administration, a temporary withdrawal of the lansoprazole may be considered in some patient.
Can lansoprazole be taken with amoxicillin?
Lansoprazole in combination with amoxicillin as dual therapy is indicated in adults for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one-year history of a duodenal ulcer) who are either allergic or intolerant to clarithromycin or in whom resistance to clarithromycin is known or suspected. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence
Can lansoprazole be taken by a patient having liver diseases?
Lansoprazole is mainly metabolized by the liver. The drug is generally well tolerated and rarely causes any complication. In a patient with severe hepatic impairment, the drug adjustment can be done by the drug titration with caution.
Can lansoprazole be given in conditions like bone fractures?
There is a moderate risk when lansoprazole or other PPIs are taken in conditions like osteoporosis or bone fractures because various studies reported that PPI therapy can be associated with increased incidences of osteoporosis.
Lansoprazole can increase the risk of osteoporosis-related fractures of hip, wrist or spine. In patients who receive high doses or multiple daily doses and long-term treatment are at increased risk of bone fractures.
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
Caution should be used in patients at risk for osteoporosis-related fractures and should be managed according to established treatment guidelines.
Can lansoprazole cause acute interstitial nephritis?
In patients who were taking PPIs including lansoprazole, acute interstitial nephritis has been observed. Acute interstitial nephritis may occur at any point during PPI therapy and is generally attributed to an idiopathic hypersensitivity reaction. Lansoprazole should be discontinued if acute interstitial nephritis develops.
Can lansoprazole provoke Clostridium difficile-associated diarrhea
PPI therapy with lansoprazole may be associated with an increased risk of Clostridium difficile associated diarrhea (CDAD), especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve
Lansoprazole use and cutaneous or systemic lupus erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including Lansoprazole.
These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematosus cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI associated SLE is usually milder than non-drug induced SLE.
Onset of SLE typically occurred within days to years after initiating treatment primarily in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Can lansoprazole give false positive results in the diagnosis of neuroendocrine tumors?
Serum chromogranin A (CgA) tumor marker levels may increase secondary to drug-induced decreases in gastric acidity which lansoprazole can cause. The increased CgA level may give false positive results in diagnostic investigations for neuroendocrine tumors.
Physicians should temporarily stop lansoprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high.
If serial tests are performed, the same commercial laboratory should be used for testing, as reference ranges between tests may vary
Can lansoprazole be given in hypomagnesemia or magnesium imbalance?
Magnesium imbalance or symptomatic hypomagnesemia or magnesium imbalance without symptoms has been rarely reported in patients treated with lansoprazole or any other PPI for 3 months at least, or even after a year of therapy. In rare cases, adverse events such as tetany, seizures, and arrhythmias can be caused.
In patients prone to magnesium imbalance such as patients taking some medications which can cause hypomagnesemia (like diuretics), caution should be used. Regular monitoring of magnesium level is advisable in such cases.
Can lansoprazole be used in pediatrics patients?
For the treatment of gastric problems in adults and children, proton pump inhibitors (PPIs) become the mostly prescribed medications.
The efficacy and safety of PPIs are established in children older than 1-year-old for the treatment of peptic conditions such as gastric ulcers, gastroesophageal reflux disease (GERD), and Helicobacter pylori infections.
However, the effectiveness of lansoprazole in treating GERD has not been established in infants. There are many reasons that support this fact such as GERD not occurring in infants, lack of complete and histologic identification of esophagitis related to GERD in infants etc.
There are certain enzymes which are immature at birth and reach adult levels of activity by 5 to 6 months after birth.
These enzymes increase the exposure and prolong the proton pump inhibitor with time. Moreover, prolonged use of PPIs for the treatment of pediatric patients has not caused cancer or significant abnormalities.
“Is Prevacid and Nexium the same thing?”
“Is there a difference between omeprazole and lansoprazole?“