Contents
- What is WPI 39 70 pill?
- Active ingredients of WPI 39 70 pill and their identification
- WPI 39 70 pill chemistry
- WPI 39 70 pill uses
- WPI 39 70 pill contraindications
- WPI 39 70 pill legal status
- What are different brand names for metronidazole?
- WPI 39 70 pills price and prescription
- WPI 39 70 pill mechanism of action
- WPI 39 70 pill side effects
- Can WPI 39 70 pill cause dependence?
- WPI 39 70 pill pharmacokinetics
- How long WPI 39 70 pill stays in the system?
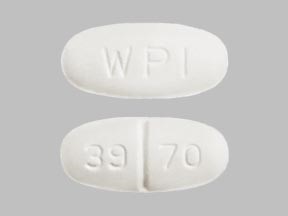
What is WPI 39 70 pill?
WPI 39 70 is an imprint on pill identified as metronidazole 500mg. It is a white colored, capsule shaped pill which is used for treating certain parasitic infections as well as anaerobic bacterial infection. Its supplier is Watson Pharmaceuticals, Inc.
- Imprint: WPI 39 70
- Strength: 500mg
- Color: White
- Shape: Capsule shaped
- Availability: Prescription only
- Drug Class: Amebicides, Miscellanious antibiotics
- Pregnancy Category: B (No proven risk in humans)
- CSA Schedule: It is not a controlled drug
- Labeler / Supplier: Watson Pharmaceuticals, Inc.
- National Drug Code (NDC): 00591-3970
- Inactive ingredients: hydroxypropyl cellulose, lactose (anhydrous), colloidal silicon dioxide, sodium starch glycolate, microcrystalline cellulose, and stearic acid.
Active ingredients of WPI 39 70 pill and their identification
WPI 39 70 contains only a single active ingredient, metronidzole, which is an oral formulation of the synthetic nitroimidazole antimicrobial, 2-methyl-5-nitro-1H-imidazole-1-ethanol
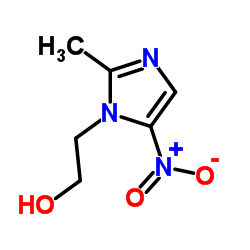
WPI 39 70 pill chemistry
WPI 39 70 contains metronidazole in 500 mg strength. The chemistry of metronidazole is as follows:
IUPAC name: 2-(2-methyl-5-nitroimidazol-1-yl)ethanol
Molecular formula: C6H9N3O3
Molecular weight: 171.156 g/mol
Molecular structure:
Drug class: Metronidazole is an organic compound belonging to the class of nitroimidazoles (imidazole ring which bears a nitro group).
WPI 39 70 pill uses
Metronidazole 500mg is the drug of choice for the treatment of symptomatic and asymptomatic trichomoniasis in both sexual partners as well as intestinal and liver amebiasis.
This drug is also indicated in treating anerobic bacteria such as Bacteroides species including the B. fragilis group, Clostridium species, Peptococcus species, Peptostreptococcus species, and Fusobacterium species. The various infections these bacteria can cause are:
- Peritonitis, intra-abdominal abscess, and liver abscess
- Skin and subcutaneous infections
- Endometritis, endomyometritis, tubo-ovarian abscess, and postsurgical vaginal cuff infection
- Bacterial septicaemia
- Bone and joint infections
- Meningitis and brain abscess
- Pneumonia, empyema, and lung abscess
- Endocarditis
WPI 39 70 pill contraindications
WPI 39 70 is contraindicated in the following individuals
- Known hypersensitivity to metronidazole or any other component of the pill
- The drug is contraindicated in patients with trichomoniasis if the mother is in the first trimester of pregnancy
- Alcohol: it can cause psychosis in such patients
WPI 39 70 pill legal status
WPI 39 70 is not a controlled drug under the controlled substances act as neither metronidazole nor any of its inactive ingredients are known to cause addiction. However, this does not mean it is an over the counter drug, and can only be obtained with a prescription.
What are different brand names for metronidazole?
Generic metronidazole is marketed by several different companies in the US. The most commonly used oral formulation is Flagyl. Other oral formulations commercially available are named RTU, Metryl, and Protostat. It is also available in topical forms and is markets as Metrocream, Metrogel, Metrogel-Vaginal, Metrolotion.
WPI 39 70 pills price and prescription
Metronidazole 500mg is a reasonably priced drug used to treat certain parasitic and bacterial infections. If you have Medicare cover or insurance cover, this drug will be most likely covered by that.
The average retail price is $17.18, which is lower for other comparable drugs. To get a discount of up to 43% on the average retail price, GoodRx can be used where it is available for $9.66.
WPI 39 70 pill mechanism of action
Metronidazole, a prodrug, contains a nitro group which readily accepts elctrons, thereby covnerting the prodrug to its active (reduced) form.
The transfer of electrons is only brought by anaerobic bacteria and occurs within the anaerobic cells. In this way this drug is only selective for anaerobic bacteria.
The active (reduced) drug then covalently binds with DNA of the bacteria, disrupting its helical structure.This will lead to cessation of bacterial nucleic acid synthesis which will lead to bacterial cell death.
Since the step which converts the prodrug to its active form occurs only in anaerobic cells, human cells are also sparred damage and hence it is a very safe drug.
WPI 39 70 pill side effects
Metronidazole may cause following side effects:
Common side effects:
- Vomiting
- GI disturbances
- Vomiting
- Loss of appetite
- Dizziness
- Runny nose, cough or sneezing
- Dry mouth
- Metallic taste
- Dark or reddish-brown urine
- Mouth irritation
- Vaginal itching
- Vaginal discharge
Serious side effects:
- Tingling and numbness in the hands and feet
- White patches or sores may appear on the lips or in the mouth
- Pain during urination
- Vision problems
- Pain behind the eyes
- Fever
- Difficulty in maintaining concentration
- Mood changes
- Behavioral changes
- Confusion
- Tremors
- Seizures
- Slurred speech
- Stiff neck
- Muscle twitching
- Joint pain (any joint maybe involved)
- Allergic reactions. These may range from a simple rash to difficulty in breathing and swelling of the face, lips, tongue, or throat (angioedema)
- The most serious side effect of metronidazole is thought to be carcinogenesis. Oral exposure in mice shows a definite increased risk of cancer, however the relationship in humans is still questionable.
Can WPI 39 70 pill cause dependence?
WPI 39 70 does not cause dependence. As a result it is not a controlled substance.
WPI 39 70 pill pharmacokinetics
Absorption: The bioavailability of metronidazole after oral intake is close to 80%.
Distribution: At any given time, the amount of drug attached to the drug is less than 20%. The volume of distribution of the drug in adults ranges from 0.51 to 1.1 L/kg.
Metabolism: The drug undergoes hepatic metabolism. Two main reactions occure in the liver. First there is side-chain oxidation yielding 1-(ß-hydroxyethyl)-2-hydroxymethyl-5-nitroimidazole and 2-methyl-5-nitroimidazole-1-yl-acetic acid.
Second reaction which takes place in the liver is glucuronide conjugation. Both the unchanged drug and the hydroxyl metabolite possess antimicrobial activity.
Excretion: The drug is eliminated by both urinary and fecal routes. Urinary route accounts for most of the elimination and fecal excretion accounts for 6% to 15% of the dose.
20% of the drug that passes through these routes is in unchanged form and the rest are its metabolites. Clearance rate of metronidazole by kidney is approximately 10 mL/min/1.73 m2.
How long WPI 39 70 pill stays in the system?
The average half-life of metronidazole is 8 hours. Since 5.5 half-lives are needed to completely clear the body of the drug, the total time needed to completely eliminate the drug from the body will be 44 hours.
However if there is hepatic or renal dysfunction this time will increase and therefore in such circumstances dose adjustment will be needed.
“Blue and white R 3059 capsule – drug class, uses, shape, size and warnings”
“Metronidazole oral and warfarin oral Drug Interactions“