Contents
- What is WPI 845 pill?
- Active ingredients of WPI 845 pill and their identification
- WPI 845 pill chemistry
- WPI 845 pill uses
- Contraindications of WPI 845 pill
- Precautions during WPI 845 pill use
- WPI 845 pill drug-drug interactions
- WPI 845 pill legal status
- What are different brand names for glipizide?
- WPI 845 pill price and prescription
- WPI 845 pill mechanism of action
- WPI 845 pill side effects
- Can WPI 845 pill cause dependence?
- WPI 845 pill pharmacokinetics
What is WPI 845 pill?
WPI 845 is an imprint on a white, round pill containing glipizide extended-release 10 mg. It is a sulfonylurea and is used in the treatment of diabetes mellitus 2. It is supplied by Watson Laboratories, Inc.
- Imprint: WPI 845
- Strength: 10mg
- Color: White
- Size: 6.00 mm
- Shape: Round
- Availability: Prescription only
- Drug Class: Sulfonylurea
- Pregnancy Category: C (Risk of teratogenicity cannot be ruled out)
- CSA Schedule: Not a controlled drug
- Labeler / Supplier: Watson Laboratories, Inc.
- Inactive Ingredients: acetyltributyl citrate, hydroxyethyl cellulose (2000 mpa.s at 1%),
hydroxypropyl cellulose (type h), methacrylic acid – methyl methacrylate copolymer (1:1), lactose monohydrate, magnesium stearate, isopropyl alcohol, ferrosoferric oxide, butyl alcohol, propylene glycol, polyethylene glycol, shellac, ammonia
Active ingredients of WPI 845 pill and their identification
WPI 845 pill contains a single active ingredient, glipizide in 10 mg strength. It is a whitish, oderless poweder whichi is is insoluble in water and alcohols, but soluble in 0.1 N NaOH. The extended-release tablets are formulated as a polymer matrix.
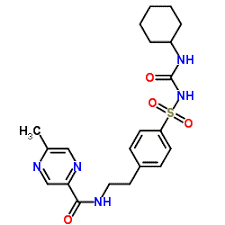
WPI 845 pill chemistry
Glipizide:
IUPAC name: N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-5-methylpyrazine-2-carboxamide
Molecular formula: C21H27N5O4S
Molecular weight: 445.538 g/mol
Molecular structure:
Drug class: Glipizide is an organic compound belonging to group known as benzenesulfonamides. These compounds contain a benzene ring which is attached to sulfonamide group through an S-linked covalent bond.
WPI 845 pill uses
Glipizide extended-release tablets are indicated in type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycemic control.
Contraindications of WPI 845 pill
Glipizide is contraindicated in patients with:
- Known hypersensitivity to glipizide or any other component of the pill
- Type 1 diabetes mellitus or diabetic ketoacidosis. These conditions should be treated with insulin.
Precautions during WPI 845 pill use
- Renal and hepatic disease: Metabolism and excretion of drug is affected, so dose should be reduced.
- Hypoglycemia: Risk of developing hypoglycemia increases with fasting, after severe or prolonged exercise, when alcohol is ingested, or when more than one glucose-lowering drug is used.
- Hemolytic anemia: Glipizide can induce anemia in individuals with G6PD deficiency as it is a sulpha containing drug.
WPI 845 pill drug-drug interactions
Those drugs which are highly bound to plasma proteins will displace glipizide from these proteins. As a result, an increase amount of free glipizide will be available which will increase the risk of developing hypoglycemia. These drugs are:
- Salicylates
- Sulphonamides
- Chloramphenicol
- Probenecid
- Monoamine oxidase inhibitors B
- Beta-adrenergic blocking agents
- Oral miconazole
These drugs lead to hyperglycemia and hence poor glycemic control
- Diuretics
- Corticosteroids
- Phenothiazines
- Thyroid products
- Estrogens and oral contraceptives
- Phenytoin
- Nicotinic acid
- Sympathomimetics
- Calcium channel blocking drugs
- Isoniazid
WPI 845 pill legal status
WPI 845 pill is not a controlled drug under the controlled substances act as neither glipizide nor any of its inactive ingredients are known to cause addiction. It is a prescription only drug.
What are different brand names for glipizide?
Glipizide is marketed by several different companies as generic glipizide. Other brand names for it are: Glucotrol, GlipiZIDE XL, Glucotrol XL
WPI 845 pill price and prescription
Metronidazole 500mg is a reasonably priced drug used in type 2 DM for glycemic control. This drug is covered by most Medicare and insurance plans. Without insurance the pill will cost you $27.65.
This is the average retail price and the actual price will depend on from where you buy the drug. On GoodRx, the cheapest and most common version of glipizide extended-release is around $9.99.
WPI 845 pill mechanism of action
Glipizide stimulates the release of insulin from beta cells in the pancreatic islets. They do so by blocking the potassium channels present at the cell surface membrane of these cells.
This will cause potassium to build up inside the cell. Eventually, there will depolarization of the cell which will cause insulin to be released.
There are two other extra-pancreatic effects of glipizide as well. It is thought to increase insulin sensitivity and decrease hepatic glucose production (gluconeogenesis). However the mechanism/s through which glipizide achieves this have not been identified.
There is wide variation how a patient will respond to this drug. Some don’t respond initially while some lose their responsiveness to sulfonylurea drugs, including glipizide.
The reason behind this is that insulin secretion will depend on the state of the pancreas. If the beta cells are completely worn out or become worn out with time, the response to glipizide will be completely absent or decrease with time respectively.
WPI 845 pill side effects
Glipizide may cause following side effects:
Common side effects:
- Nausea, vomiting, Diarrhea
- Bloating, flatulence
Serious side effects. Immediately see your doctor if these appear:
- Dizziness
- Skin changes: red or itchy skin, rash, hives, blisters or any other skin reaction
- Tremors (Uncontrollable shaking)
- Signs of jaundice: dark urine, light-colored stools, yellowish discoloration of the eyes or skin
- Mental or mood changes
- Fever
- Sore throat
- Pain in the upper right area of the abdomen
- Unusual bleeding or bruising
- Sudden weight gain
- Psychosis
- Seizures
- Angioedema (swelling of the eyes, face, lips, tongue, or throat)
Can WPI 845 pill cause dependence?
WPI 845 pill does not cause dependence. As a result it is not a controlled substance.
WPI 845 pill pharmacokinetics
Absorption: The bioavailability of fenofibrate after oral intake is high, around 90%.
Distribution: The plasma protein binding of fenofibrate is very high and is around 98% to 99%. The volume of distribution of glipizide is found to be 10L.
Metabolism: Glipizide undergoes hepatic hydroxylation. These aromatic hydroxylation products have no hypoglycemic activity.
Hepatic metabolism also yields another metabolite, an acetylamino-ethyl benzene derivative which is reported to have 1/10 to 1/3 as much hypoglycemic activity as the parent drug.
Excretion: 10% of the drug is excreted in unchanged form in urine and feces. The remaining 90% of excreted drug is in the form of hepatic metabolites. 80% is eliminated in urine while remaining 10% is eliminated in feces.
How long WPI 845 pill stays in the system?
The half-life of glipizide is variable and ranges from 2 hours to 5 hours. Therefore the time needed by the body to completely get rid of the drug ranges from 11 hours in some individuals to 27.5 hours in others.
“What are the side effects of metformin and glipizide?”
“377 pill: Drug class, ingredients, uses, dosage and side effects“