Contents
- What is Bupropion?
- Bupropion generic and brand name
- Why is Bupropion prescribed
- Chemical information of the drug
- Available strength of Bupropion
- How Bupropion works?
- What are the recommended doses of Bupropion?
- When should I discontinue, withhold or modify the dose of Bupropion?
- The pharmacokinetic properties of bupropion
- Which pregnancy category (A; B; C; D; X) has been assigned to Bupropion?
- How to take Bupropion?
- How to store the drug?
- How to dispose the medicine?
- Does Bupropion has approval from government / FDA /or any other related agencies?
- Other uses of the drug
- What special dietary precautions should I follow?
- What special precautions should I follow? /What should I avoid while using Bupropion
- Bupropion possible side effects
- What should I do in case of overdose?
- What should I do in case of missed a dose?
- Bupropion drug interactions
- Does Bupropion have any interaction with Diseases?
What is Bupropion?
- Bupropion is antidepressant drug that is used for the treatment of depression and seasonal affective disorder.

Bupropion generic and brand name
- The drug is available under generic name Bupropion and brand names such as Wellbutrin and Zyban.
- In addition, Bupropion is also known as Amfebutamone.
- Bupropion was discovered in 1969 by Nariman Mehta.
- Bupropion is manufactured and marketed by
- Bupropion is also manufactured by Sanofi–Aventis under the brand name Aplenzin.
What is the source of drug (natural or synthetic)?
- Bupropion is a synthetic (man-made) second generation antidepressant pharmaceutical agent.
Why is Bupropion prescribed
- Bupropion plays a key role in the treatment of major depressive disorder.
- Bupropion is used to treat seasonal affective disorders (depression that occurs during the same season each year).
- Bupropion is also prescribed for the treatment of Smoking cessation.
- Bupropion is also used in the treatment of obesity.
- Bupropion is often used to treat attention deficit hyperactivity disorder.
- However, Bupropion does not effective in the treatment of chronic low back pain.
Pharmacophore structure: Information about the chemical structure of the drug
Bupropion chemically belongs to the class of organic compounds which are known as Acetophenones characterized by acetophenone structure. The detailed chemical classification of Bupropion is described below:
| Kingdom | Organic compounds |
| Super Class | Benzenoids |
| Class | Benzene and substituted derivatives |
| Sub Class | Acetophhenones |
| Direct Parent | Acetophhenones |
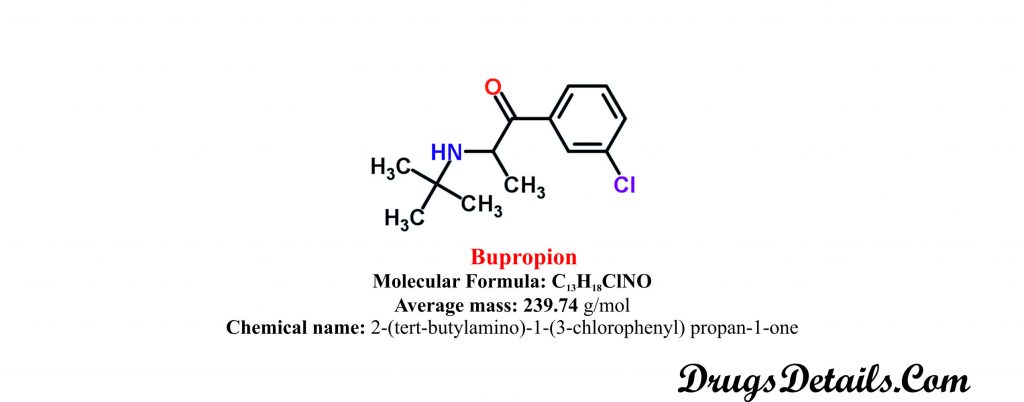
Chemical information of the drug
- Bupropion is aminoketone and available as a monohydrochloride salt as well as in form of monohydrobromide salt.
- It is a synthetic pharmaceutical aromatic homomonocyclic compound with a molecular formula C13H18ClNO.
- The molecular weight of the compound is 74 g/mol.
- Chemically, Bupropion is monpohydrochloric or monohydrobromide salt of 2-(tert-butylamino)-1-(3-chlorophenyl) propan-1-one.
- Bupropion is white in color, crystalline, bitter in taste, very hygroscopic and has a water solubility of 312 mg/mL.
- Bupropion is soluble in methanol, ethanol, benzene and acetone.
- The melting point of Bupropion is 233-234°C.
Available strength of Bupropion
- Bupropion is available in immediate-release, sustained-release and extended-release tablet form for oral administration.
- Bupropion HCl tablet (WELLBUTRIN®) is available in dosage strength of 75 and 100 mg/tablet.
- 75 mg: yellow-gold in color, biconvex, round tablets and printed with “WELLBUTRIN 75”.
- Inactive ingredients: hydroxypropyl cellulose, hypromellose, microcrystalline cellulose, polyethylene glycol, talk, titanium dioxide, D&C Yellow No. 10 Lake and FD&C Yellow No. 40 Lake.
- 100 mg: red in color, biconvex, round tablets and printed with “WELLBUTRIN 100”.
- Inactive ingredients: hydroxypropyl cellulose, hypromellose, microcrystalline cellulose, polyethylene glycol, talk, titanium dioxide, FD&C Red No. 40 Lake and FD&C Yellow No. 6 Lake.
- Bupropion HCl extended-release tablet (WELLBUTRIN XL®) is available in dosage strength of 150 and 300 mg/tablet.
- WELLBUTRIN XL 150 and 300 mg tablets are creamy-white to pale yellow in color, round and imprinted with “WELLBUTRIN XL 150” and “WELLBUTRIN XL 300” respectively.
- WELLBUTRIN XL contains Bupropion HCl as active ingredient and ethylcellulose, polyvinyl alcohol, polyethylene glycol, povidone, glyceryl behenate, methacrylic acid copolymer dispersion, silicon dioxide and triethyl citrate as inactive ingredients.
- Bupropion HCl sustained-release tablet (WELLBUTRIN SR®) is available in dosage strength of 100mg, 150mg and 200mg.
- 100 mg: blue in color, biconvex, round, film-coated tablets printed with “WELLBUTRIN SR 100”.
- Inactive ingredients: hypromellose, microcrystalline cellulose, polyethylene glycol, carnauba wax, cysteine hydrochloride, magnesium stearate, polysorbate 80, titanium dioxide, FD&C Blue No. 1 Lake.
- 150 mg: purple in color, biconvex, round, film-coated tablets printed with “WELLBUTRIN SR 150”.
- Inactive ingredients: hypromellose, microcrystalline cellulose, polyethylene glycol, carnauba wax, cysteine hydrochloride, magnesium stearate, polysorbate 80, titanium dioxide, FD&C Blue No. 2 Lake and FD&C Red No. 40 lake.
- 200 mg: light pink in color, biconvex, round, film-coated tablets printed with “WELLBUTRIN SR 200”.
- Inactive ingredients: hypromellose, microcrystalline cellulose, polyethylene glycol, carnauba wax, cysteine hydrochloride, magnesium stearate, polysorbate 80, titanium dioxide and FD&C Red No. 40 lake.
- Bupropion HBr extended-release tablet (APLENZIN®) is available in different dosage strength of 174mg, 348mg and 522mg.
- APLENZIN 174mg, 348mg and 522mg tablets are white to off-white in color, round and imprinted with “BR” over “174”, “BR” over “348” and “BR” over “522” respectively.
- APLENZIN contains Bupropion hydrobromide as active ingredient and ethylcellulose, glyceryl behenate, polyvinyl alcohol, polyethylene glycol, povidone, and dibutyl sebacate as inactive ingredients. In addition, 174 and 348 mg tablets also contain carnauba wax.
How Bupropion works?
Bupropion mechanism of action:
- Bupropion selectively inhibits the neural uptake of dopamine, serotonin and norepinephrine.
- Bupropion is somewhat weak inhibitor of neural uptake of serotonin and norepinephrine as compared to other tricyclic antidepressant.
- Bupropion has main effect on neural uptake of dopamine. However, it is chemically unrelated to tetracyclic or tricyclic selective serotonin re-uptake inhibitor.
- Increased norepinephrine can attenuate nicotine withdrawal symptoms.
- The increased dopamine at neuronal sites possibly diminishes nicotine longing and the urge to smoke.
- Bupropion exhibits moderate anticholinergic (block the neurotransmitter acetylcholine in central and peripheral nervous system) effect.
What are the recommended doses of Bupropion?
- The prescribed dose of Bupropion varies depending upon the age and diseased state of the patient.
- Depression
Bupropion hydrochloride
- Immediate release tablets
Initial dose: 100 mg twice a day orally for 3 days.
Maintenance dose: 100 mg thrice a day orally (maximum up to 450 mg in 4 divided doses per day).
- Sustained release tablets
Initial dose: 150 mg once a day orally in morning for 3 days.
Maintenance dose: 150 mg twice a day orally (maximum up to 200 mg twice daily).
- Extended release tablets
Initial dose: 150 mg once a day orally in morning for 4 days.
Maintenance dose: 300 mg once a day orally (maximum up to 450 mg once daily).
Bupropion hydrobromide
- Extended release tablets
Initial dose: 175 mg once a day orally in morning for 4 days.
Maintenance dose: 348 mg once a day orally (maximum up to 522 mg once daily).
- Seasonal affective disorder
Bupropion hydrochloride
- Extended release tablets
Initial dose: 150 mg once a day orally in morning for 7 days.
Maintenance dose: 150-300 mg once a day orally.
Bupropion hydrobromide
- Extended release tablets
Initial dose: 175 mg once a day orally in morning for 7 days.
Maintenance dose: 348 mg once a day orally.
- Smoking cessation
Bupropion hydrochloride
Initial dose: 150 mg once a day orally in morning for 3 days.
Maintenance dose: 150 mg twice a day orally for 7 to 12 weeks.
- Dose adjustment in liver impairment:
Bupropion hydrochloride
- Immediate release tablets
Mild: reduction in dose or dosing frequency.
Moderate to severe: 75 mg in every 48 hours orally.
- Sustained release tablets
Mild: reduction in dose or dosing frequency.
Moderate to severe: 100 mg in every 48 hours orally.
- Extended release tablets
Mild to moderate: reduction in dose or dosing frequency.
Severe: 150 mg in every 48 hours orally.
Bupropion hydrobromide
- Extended release tablets
Moderate to severe: 175 mg in every 48 hours orally.
- Dose adjustment in renal impairment: reduction in dose or dosing frequency may necessary.
When should I discontinue, withhold or modify the dose of Bupropion?
- The usual dosing of the drug may vary depending upon the efficiency and side effects of the drug in a particular individual.
- Do not use the medicine if you are allergic to Bupropion or any of the ingredients present in the Bupropion product.
- Bupropion use with precaution in case of renal and hepatic impairment.
- Bupropion may be discontinued in case of eating disorders (such as bulimia and anorexia nervosa).
- Bupropion may be withheld if you are taking monoamine oxidase inhibitors (MAOIs) such as Isocarboxazid, Linezolid, Phenelzine, selegiline and Tranylcypromine.
- Bupropion is contraindicated with the use of other drugs such as Amantadine, Clopidogrel (inhibitor of CYP2B6), Cyclophosphamide and Efavirenz.
- Bupropion is contraindicated with the medications of diabetes, medications of irregular heartbeats (Flecainide and Propafenone), mental illness (Haloperidol, Tisperidone and Thioridazine), seizures (Carbamazepine, Phenobarbitaland Phenytoin), oral steroids (Dexamethasone, Methylprednisolone and Prednisone) and beta blockers (Atenolol, Metoprolol, Nadolol, Propranolol and Labetalol).
- Bupropion is also contraindicated with other antidepressants (such as desipramine, desipramine, fluoxetine, fluvoxamine, nortriptyline, paroxetine, and sertraline), sleeping pills and sedatives.
The pharmacokinetic properties of bupropion
- The bio-availability of Bupropion in human has not been determined. Laboratory animal studies suggest that the bioavailability of Bupropion in rat and dogs is ranged from 5% to 20%.
- It has been observed that following two single dose of 150 mg every 12 hours, the maximum (or peak) plasma concentration (136ng/mL) is achieved in 180 minutes in the fasted state.
- Following absorption the majority (84%) of the drug is bound to plasma proteins.
- The drug is mainly metabolized by the hepatic enzyme CYP450
- CYP450 1A2, CYP450 2A6, CYP450 2C9, CYP450 3A4, CYP450 2E1 and CYP450 2C19 may also involved in the metabolism of Bupropion.
- Bupropion is primarily metabolised into hydroxybupropion and amino alcohol isomers threohyrobupropion and erythrobupropion active compound through tert-butyl group hydroxylation and carbonyl group reduction of Bupropion.
- The average median half-life of Bupropion is 24
- Bupropion is primarily excreted in the urine (87% in the form of metabolites and 0.5% as uncharged drug) and in feces (10%).
- The average steady state volume of distribution of the drug from a single dose of 150mg is 1950 L.
Which pregnancy category (A; B; C; D; X) has been assigned to Bupropion?
- The Bupropion is classified by US FDA pregnancy category: C
- Due to lack of adequate and well-controlled studies the use of Bupropion in pregnant women is contraindicated and recommended only when benefit justifies the risk.
- Laboratory animal studies have shown no specific damage to the fetus.
- Studies support the excretion of the Bupropion in human breast milk at very low levels but it not causes any severe damage to breastfed infants.
- Despite these facts caution should be exercised when taking Bupropion.
How to take Bupropion?
- Bupropion is available in immediate, sustained and extended release tablets form for oral administration by mouth with or without food.
- Extended release tablets should be taken once a day in morning.
- Bupropion regular tablet is usually taken 3 times and sustained release tablets taken twice in a day orally.
- Do not chew split or crush Bupropion sustained and extended release tablets, swallow whole.
- In case of seasonal affective disorder, medication continuing throughout winter and stopping in early spring.
- It is also recommended to take drug at almost the same time every day.
- Do not change the dose of the drug as prescribed by your doctor. Since, your doctor may decrease the dose of Bupropion depends upon adverse side effects or medication response.
- If you have any queries about the drug immediately consult to your doctor to explain any part you do not understand.
How to store the drug?
- Bupropion is stored at room temperature 25°C (77°F).
- Brief excursion period is permitted to 15-30°C (59-86°F).
- Store the medicine away from direct sunlight, excess heat and moisture.
- The drug should be kept away from children and pets.
How to dispose the medicine?
- Throw away unused and opened, outdated or no longer used container.
- Also dispose the old medicine after the expiration date.
- Consult your pharmacist or local waste disposal company for proper disposal.
- Bupropion has received its official approval from US Food and Drug Administration (FDA) in December, 1985 for the treatment of depression.
- Bupropion sustained release formulation was approved in 1996 by U.S. FDA.
- Bupropion is also approved by U.S. FDA to treat smoking cessations in 1997.
- A new sustained release formulation known as Wellbutrin XL is also approved by U.S. FDA in 2003 for the treatment of depression and also for the treatment of seasonal affective disorder in 2006.
- New formulation i.e. Bupropion hydrobromide salt is also approved by FDA in April 2008.
Other uses of the drug
- Bupropion is also used in the treatment of bipolar disorder (a disease that cause episode of depression, mania and other abnormal moods).
- Bupropion may be useful to treat inflammatory bowel disease.
- Bupropion may also be used for other uses not listed here. It is advisable to ask your doctor or pharmacist for more detailed information regarding its use.
What special dietary precautions should I follow?
- Take diet as prescribed by your doctor otherwise follow usual diet.
What special precautions should I follow? /What should I avoid while using Bupropion
- Before taking Carvedilol, tell your doctor about your medical history preferentially if you have any kind of kidney or liver disease, diabetes and high blood pressure.
- Consult with your doctor and pharmacist if you are taking any prescription and non-prescription medications or herbal products.
- Consult to your doctor if you have a tumour in spine or brain or ever had a heart attack.
- Carvedilol medication should not be stopped suddenly and avoid to skip the dose.
- Avoid using machinery requiring alertness or clear vision as well as driving because the use of Bupropion may make you drowsy.
- Check blood pressure before starting the medication of Bupropion and regularly because it may increase the blood pressure.
- Bupropion should not take too close to bedtime if you feel sleeplessness.
- Use of alcohol or alcoholic beverages should be avoided because Alcohol consumption can also enhance the side effects of the drug.
Bupropion possible side effects
In addition to the associated benefits, Bupropion also is accompanied with the side effects some of which are more common, others less common whereas some that fade away with time while you take the drug. It is always recommended to consult a doctor if you encounter any of the side effects.
Most common side effects caused by Bupropion are as follows:
- Anxiety
- Changes in your sense of taste
- Constipation
- Difficulty falling asleep or staying asleep
- Dizziness
- Dry mouth
- Headache
- Loss of desire for food
- Nausea
- Sore throat
- Stomach pain
- Uncontrollable shaking of a part of the body
- Vomiting
- Weight loss
There are some adverse effects that fade away while consuming the drug with time. These symptoms do not require any medical attention, but if these symptoms persist immediately contact to your doctor.
- Alteration in sense of taste
- Blurred vision
- Constipation
- Decrease in appetite
- Dizziness
- Drowsiness
- Increased sweating
- Recurrent need to urinate
- Stomach or abdominal pain
- Trembling
- Unusual weight loss
Bupropion may cause some serious side-effect which requires immediate medical attention are as follows:
- Confusion
- Hallucinating (seeing things or hearing voices that do not exist)
- Muscle or joint pain
- Seizures
- Unbalanced, rapid or pounding heartbeat
- Unreasonable fears
Stop taking Bupropion and find urgent medical treatment if these symptoms are observed:
- Fever
- Difficulty breathing or swallowing
- Hives
- Hoarseness
- Itching
- Pain in chest
- Rash or blisters
- Swelling of the face, throat, tongue, lips, eyes, hands, feet, ankles, or lower legs
Besides these, Bupropion may also be associated with some other side effects. These include:
- Cardiovascular effects: tachycardia, hypertension, cardiac arrhythmias, migraine, palpitation, edema, postural hypotension, stroke and vasodilation.
- Dermatologic effects: urticaria, dry skin, pruritus, alopecia, erythrma multiforme and photosensitivity.
- Gastrointestinal effects: vomiting, constipation, nausea, diarrhoea, abdominal pain, stomatitis, flatulence, gustatory disturbance, gingivitis, gum irritation, oral edema, increased salivation and mouth ulcers.
- Hypersensitivity effects: allergic reactions and fever with rashes.
- Musculoskeletal effect: arthritis, arthralgia, myalgia, neck pain, musculoskeletal chest pain.
- Nervous system effects: sedation, tremor, akathisia, parathesia, dyskinesia, myoclonus, dystonia, somnolence and abnormal cordination.
- Ocular effects: diplopia or blurred vision.
- Psychiatric effect: insomnia, agitation, abnormal dreams, anxiety, euphora, hypomania, thinking abnormalities, dysphoria, suicidal ideation, depersonalizations, frigidity, hostility and paranoia.
- Respiratory effects: rhinitis, nasopharyngitis, bronchitis, epistaxis, sinusitis, increased cough, upper respiratory tract infection and bronchospasm.
- Genitourinary effects: decreased sexual function, menstrual complaints, vaginal haemorrhage, urinary tract infections, impotence, prostate disorder, testicular swelling, nocturia and polyuria.
What should I do in case of overdose?
- If you overdose the drug contact with your doctor or pharmacist for symptomatic and supportive measures.
- Overdose of Bupropion may cause seizure, loss of consciousness and rapid heartbeat.
What should I do in case of missed a dose?
- In case of missed dosage, take it as soon as you remember and maintain a regular dosing schedule.
- Skip the missed dose if it is almost time for your next scheduled dose.
- Keep in mind to not use a double dose to make up a missed dose.
Bupropion drug interactions
Does Bupropion have any interaction with drugs? Bupropion may interact with other medications, vitamins or herbs. Care should be taken when you are taking these drugs together.
Systematic studies on Bupropion metabolism following concomitant administration with other drugs have revealed that other drugs may affect Bupropion clinical activity.
It has been observed that bupropion is primarily metabolized by the CYP2B6 isoenzyme to 11 hydroxybupropion, therefore any drug that affect CYP2B6 isoenzyme (e.g. orphenadrine and cyclophosphamide) can potentially interact with Bupropion.
This interaction can be harmful or prevent the drug from working well. Some drugs when given concomitantly with Bupropion can cause serious side effects. These drugs and Bupropion should not be taken simultaneously. Examples of these drugs include:
- Cimetidine: Concomitant oral administration of Cimetidine (150mg) and Bupropion (800mg) results in 16% and 32% increases in the AUC and Cmax of threohydrobupropion and erythrohydrobupropion.
- Drugs Metabolized by Cytochrome P450IID6 (CYP2D6): Drugs such as beta-blockers, antidepressants (SSRIs, many tricyclics), antiarrhythmics, and antipsychotics are primarily metabolized through CYP2D6 isoenzyme. Since, Buupropion and hydroxybupropion are inhibitors of the CYP2D6 isoenzyme, it can interfare with metabolization of these drugs. In clinical trials it has been observed that daily doses of Bupropion ( 150 mg twice daily) increased the Cmax, AUC, and t1/2 of drugs that metabolized through CYP2D6 isoenzyme. Therefore, administration of Bupropion in combination with other drugs that are metabolized by CYP2D6 are advised to be taken with caution. Apart from this it is also recommended to initiate these drugs at the lower end of the dose range. Some common examples of these drugs are antidepressants (e.g., desipramine, nortriptyline, paroxetine, imipramine, fluoxetine, sertraline), beta-blockers (e.g., metoprolol), antipsychotics (e.g., risperidone, haloperidol, thioridazine) and Type 1C antiarrhythmics (e.g., flecainide, propafenone,) etc.
- Levodopa and Amantadine: Clinical studies have suggested that Bupropion in combination with Levodopa / Amantadine showed higher incidence of adverse experiences. In this view, it is recommended to take caution while taking this combinations and additional precaution such as small initial doses and small gradual dose increases should be undertaken.
- Anti-seizure drugs: It is recommended that concurrent administration of Bupropion with drugs that lower seizure threshold (e.g., antidepressants, antipsychotics, systemic steroids, theophylline, etc.) should be undertaken with extreme caution.
- MAO Inhibitors: Bupropion co-administration with MAO inhibitors (isocarboxazid, selegiline, moclobemide, linezolid, phenelzine, procarbazine, methylene blue, rasagiline, tranylcypromine) can cause serious (possibly fatal) drug interaction. It is generally recommended to avoitconcurrent use of MAO inhibitors during treatment with Bupropion.
- Alcohol: It has been observed that concomitant use alcohol during treatment with Bupropion can cause adverse neuropsychiatric events or reduced alcohol tolerance in patients. The consumption of alcohol during treatment with Bupropion is prohibited or should be minimized or avoided
Does Bupropion have any interaction with Diseases?
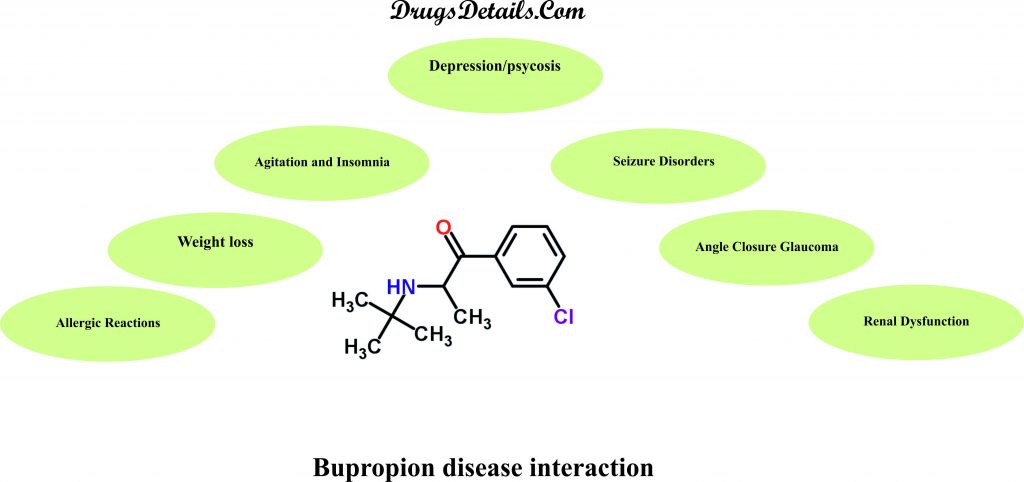
It has been observed that following medical conditions (disease) may interact with Bupropion:
- Agitation and Insomnia: A significant number of patients upon initiation of Bupropion therapy have shown agitation, restlessness, anxiety, and insomnia. Some of these patients required treatment with sedatives/hypnotic drugs while other required discontinuation of therapy. Hence, proper monitoring of the patients is required for these associated side effects upon treatment with the drug.
- Hepatic impairment: The metabolism of Bupropion is primarily carried out by the liver. The patients of hepatic impairment show increased half life, exposure and accumulation of Bupropion and its metabolites. Bupropion therapy should be administered cautiously in patients with impaired hepatic function (mild to moderate hepatic cirrhosis) and require reduced dose and/or frequency. However, patients with severe hepatic cirrhosis require extreme caution while given Bupropion therapy and a reduced dosage which should not exceed 75 mg per day.
- Angle Closure Glaucoma: Bupropion may result in an increased intraocular pressure due to its dilating effect on pupil of the eyes and thereby causing angle-closure (narrow angle) glaucoma in patients with anatomically narrow angles. It is therefore recommended to examine the patients for determination of their susceptibility to angle closure and treatment given to them for the prevention of the condition before initiating Bupropion therapy. The use of the drug should be avoided in case of patients with untreated anatomically narrow angles.
- Renal Dysfunction: Bupropion metabolites including its active metabolites are excreted through the kidneys. The patients with renal impairment may exhibit accumulation of Bupropion and its metabolites to a higher extent. The administration of Bupropion should be done cautiously in such patients with a reduced dosage and frequency. A close monitoring of the patients is required for possible adverse effects indicating high drug or metabolite levels.
- Seizure Disorders (Bulimia, Anorexia Nervosa, Alcoholism, Drug Abuse/Dependence, CNS Disorder, Diabetes Mellitus):Bupropion administration may result in seizures in a dose-dependent which increases dramatically at higher dosages. It is therefore recommended to avoid the use of Bupropion in patients with underlying seizures disorders due to variation in individual capacity for the metabolization of the drug and seizure incidence with the drug dose reduction. The use of the drug also increases the incidence of seizures in patients who have or previously had bulimia or anorexia and hence not recommended. Bupropion therapy should be done with caution in patients with other predisposing factors for seizures that include excessive use of or sudden withdrawal from alcohol; diabetes treated with oral hypoglycemic agents or insulin; underlying neurologic abnormalities (brain damage, head trauma or CNS tumor); addiction to cocaine, or opiates; and concomitant use of medications that lower seizure threshold.
- Depression: The administration of Bupropion during the first few months of therapy or during dose change may result in exacerbation of associated symptoms and emergence of suicidal thoughts and behavior in patients with depression and other psychiatric disorders. Such patients require close monitoring for worsening of their symptoms, suicidal thoughts or changes in their behavior. The therapy should be withdrawn in case these symptoms are persistent and/or occur abruptly.
- Psychosis: Bupropion administration may trigger or exacerbate the psychotic symptoms such as hallucinations, delusions, and confusion. Patients of bipolar disorder characterized by depression may show a change from the state of depression to mania (abnormally elevated mood state) or hypomania. Close monitoring of patients with psychotic disorders during Bupropion therapy is recommended to check for worsening of symptoms and the dosing adjusted either alone or in combination with other medications (e.g., tranquilizers) as required.
- Weight Loss: Bupropion therapy may be associated with changes in weight including both weight gain and weight loss. However, weight loss is much more prominent in comparison to weight gain. The frequency of weight loss greater than 5 pounds is around 28% and usually unacceptable in patients with malnutrition, anorexia or excessive weight loss. A close monitoring for any alteration in weight is necessary during treatment with Bupropion.
- Allergic Reactions:Use of Bupropion is not recommended in case of people who are allergic or hypersensitive to Bupropion or any of its constituents. Clinical studies indicate medical treatment in case of development of anaphylactic/anaphylactoid reactions characterized by symptoms including angioedema, pruritus, urticaria, dyspnea, erythema multiforme, Stevens-Johnson syndrome, and anaphylactic shock. It is recommended to stop the use of the drug and consult a doctor in case of occurrence of allergic or anaphylactoid/anaphylactic reactions during drug therapy.
Where can I get more information?
Your pharmacist can provide more information about Bupropion.
Clinical research and current scenario of the drug
- Studies involving major depressive disorder (MDD) and other psychiatric disorders indicate the increased risk of suicidal thinking and behavior associated with the use of antidepressants (Bupropion) in children, adolescents, and young adults compared to placebo.
- Animal studies that the coadministration of Bupropion and monoamine oxidase (MAO) inhibitors is potentially hazardous.
- Clinical studies indicate no effect on the pharmacokinetics of Bupropion or its metabolites in depressed patients with left ventricular dysfunction compared with healthy volunteers.
- Pharmacokinetic studies in healthy male and healthy female subjects indicate no sex-related variation in the peak plasma concentrations of Bupropion.
- Studies reveal no significant differences in Cmax, half-life, AUC, or clearance of Bupropion or its active metabolites between smokers and nonsmokers after oral administration of a single 150-mg dose of drug.
- Placebo-controlled studies in adult’s inpatients and outpatients with major depressive disorder (MDD) indicate the efficacy of Bupropion in the treatment of major depressive disorder.
- Clinical studies involving normal volunteers, in subjects with a history of multiple drug abuse, and in depressed subjects reveal an increase in motor activity and agitation/excitement upon Bupropion use.
- No studies document the safety and efficacy of Bupropion in the pediatric population.
- Clinical data suggest a higher incidence of treatment-emergent hypertension in case of patients treated with the combination of sustained-release Bupropion and nicotine transdermal system (NTS) as an aid to smoking cessation.
- Studies indicate no differences in responses to Bupropion between the elderly and younger patients; however, a greater sensitivity is associated with older subjects.
References from chemical, biological and toxicological databases
- Bupropion – Wikipedia, the free encyclopedia. https://en.wikipedia.org/wiki/Bupropion
- DrugBank: Bupropion. http://www.drugbank.ca/drugs/DB01156
- Bupropion: MedlinePlus Drug Information. https://www.nlm.nih.gov/medlineplus/druginfo/meds/a695033.html
- bupropion | C13H18ClNO | ChemSpider. http://www.chemspider.com/Chemical-Structure.431.html’
- Bupropion (By mouth) – National Library of Medicine. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0009361/?report=details
- Bupropion: pharmacology and therapeutic applications. www.ncbi.nlm.nih.gov/pubmed/17009913
- 15 Years of Clinical Experience With Bupropion HCl: From Bupropion to Bupropion SR to Bupropion XL. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1163271/
- Why isn’t bupropion the most frequently prescribed antidepressant? http://www.ncbi.nlm.nih.gov/pubmed/15889947
- Bupropion: a review of its mechanism of antidepressant activity. http://www.ncbi.nlm.nih.gov/pubmed/7665537
- A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC514842/
- Bupropion: a review of its use in the management of major depressive disorder. http://www.ncbi.nlm.nih.gov/pubmed/18370448
- Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2416755/
