Contents
Omeprazole vs Esomeprazole
Gastric acid has been known to play an imperative role in the normal upper gastrointestinal functions, including calcium and iron absorption, protein digestion, and also provide protection against bacterial infections to some extent.
However, inappropriate level of gastric acid is the root cause of several widespread pathological conditions such as gastroesophageal reflux disease (GERD), gastritis, or peptic ulcer disease affecting millions of people.
Although a variety of medications are available to combat such conditions but the most efficient drugs under this category belong to the group known as proton pump inhibitors.
Proton pump inhibitors (PPIs) are the drugs that act on the parietal cells in the lining of the stomach that secretes gastric acid. The drugs namely, Omeprazole (Prilosec) and Esomeprazole (Nexium) fall under this drug group and are the most commonly used PPIs.
The two drugs exhibit similarities to a greater extent. Both the drugs belong to the class of medications known as proton pump inhibitors and exhibit similar mechanism of action (prevention of acid secretion by the stomach).
Besides, the drugs display same drug interaction, associated side effects and are acted upon by the body in the similar fashion.
However, the drugs differ in their chemical composition, bioavailability and extended adverse effects. Esomeprazole exhibit greater bioavailability, efficacy and extended array of side effects over Omeprazole.
Although studies indicate a greater antimicrobial activity of esomeprazole in eradication of H.pylori than the omeprazole, further studies are required to establish the effectiveness of Esomeprazole over Omeprazole in decreasing the amount of acid produced in the stomach.
Omeprazole
Omeprazole is a highly effective medication that belongs to the class of drugs known as proton pump inhibitors and blocks gastric acid secretion.
Omeprazole is a pro drug (a biologically inactive agent that produces a drug upon metabolization in the body) that was approved by FDA in September 1989.
It is one of the Essential Medicines in the World Health Organization’s List of the most important medications required in a basic health system.
Omeprazole is available as prescription and over the counter forms (OTC). The generic name of the drug is Omeprazole, Omeprazole/Sodium bicarbonate and is available under several brand names such as Prilosec, Zegerid, Prilosec OTC, Zegerid OTC.
Omeprazole was first marketed in the United States in 1989 by Astra AB, now AstraZeneca, under various brand names such as Losec, Antra, Gastroloc, Mopral, Omepral, and Prilosec.
Besides, Omeprazole is marketed as Zegerid by Santarus, Prilosec OTC by Procter & Gamble and Zegerid OTC by Schering-Plough, Omez by Dr. Reddy’s Laboratories.
Omeprazole is a polycyclic aromatic compound that belongs to a class of organic compounds known as sulfinylbenzimidazoles (containing a sulfinyl group attached at the position 2 of a benzimidazole moiety).
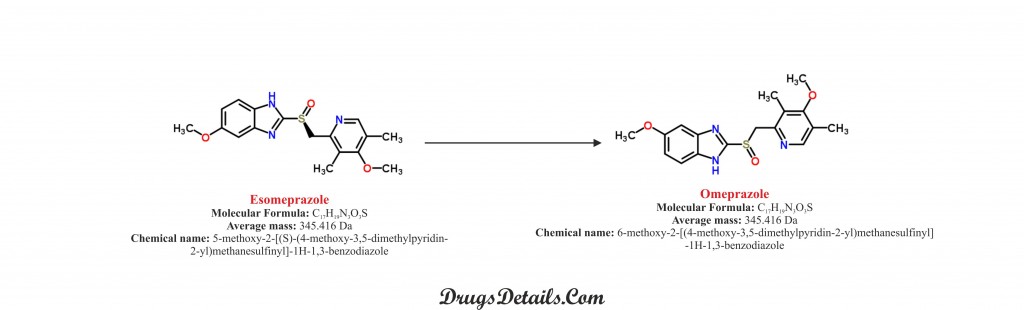
The drug is a synthetic pharmaceutical organic compound named as 6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1H-1,3-benzodiazole.
Omeprazole is a racemic mixture of R- and S- isomers in the proportion 1:1. Omeprazole is a white to off-white crystalline powder with a molecular weight of 345.41606 g/mol and molecular formula C17H19N3O3S.
Omeprazole is slightly soluble in acetone and isopropanol, freely soluble in methanol and ethanol, and very slightly soluble in water. The melting point of Omeprazole is 155 °C.
Its bioavailability ranges from 30-76%. The absorption of the drug is rapid with peak plasma levels reaching within 0.5 – 3.5 hours. Approximately 95% of the drug after absorption is bound to plasma proteins.
Omeprazole is extensively metabolized by the hepatic system via cytochrome P450 (CYP) enzyme system (CYP2C19, CYP3A4). The drug is eliminated mainly in urine as metabolites (about 80%) and the remaining 20% as feces.
Its biological half life varies from 1–1.2 hours. The drug is a benzimidazole with selective and irreversible proton pump inhibition activity.
Omeprazole results in the suppression of gastric acid secretion by formation of a stable disulfide bond with the sulfhydryl group of the hydrogen-potassium (H+/ K+) ATPase enzyme system present at the secretory surface of parietal cells in the stomach and thus inhibiting the final step in the acid production i.e., transport of hydrogen ions (via exchange with potassium ions) into the gastric lumen.
Prescription Omeprazole is available as a delayed-release capsule (10, 20 and 40 mg), and packets of delayed-release granules (20 and 40 mg) for suspension whereas nonprescription (over-the-counter) Omeprazole comes in the form of a delayed-release capsule or tablet (20 mg) to take by mouth.
The dose of the drug depends upon the disease, age and weight. The active ingredient in the delayed-release capsule and delayed-release granule (oral suspension) are Omeprazole and Omeprazole magnesium respectively. The drug is taken atleast one hour before meals.
The drug (prescription) is prescribed either alone or in combination with other medications in therapy of gastroesophageal reflux disease (GERD), acid-induced inflammation, erosive esophagitis, stomach and duodenum ulcers, Zollinger-Ellison Syndrome (gastrin-producing tumour), and heartburn.
Besides, Omeprazole is also recommended for the treatment and prevention of reoccurrence of ulcers caused by a certain type of bacteria (H. pylori) in combination with other antibiotics such as Clarithromycin and Amoxicillin.
The Over-the counter (OTC) form is used for treatment of frequent heartburn. The drug may cause allergic reactions (difficulty breathing; hives; swelling of your tongue, lips, throat, or face), common side effects (stomach pain, diarrhea, vomiting, gas, dizziness, nausea, headaches, rash), serious side effects (severe stomach pain, diarrhea; seizure (convulsions); kidney problems; symptoms of low magnesium (including fast or uneven heart rate, dizziness, muscle spasms in hands and feet, tremors (shaking) or jerking muscle movements, confusion, muscle cramps, cough or choking feeling).
Omeprazole is classified by US FDA pregnancy category: C. The drug may interact with a number of drugs including Bosentan; Clopidogrel; Cilostazol; Cyclosporine; Diazepam (Valium); Digoxin; Disulfiram (Antabuse); Erlotinib; Iron-containing medicines; Mycophenolate mofetil; Tacrolimus; Methotrexate; Phenytoin; St. John’s Wort; Antibiotics such as Clarithromycin, Ampicillin, Rifampin, Amoxicillin; Antifungal medicine including Voriconazole, Ketoconazole; HIV Or AIDS medication–Atazanavir, Nevirapine, Saquinavir, Nelfinavir, Nelfinavir, or Ritonavir; Blood thinner such as Warfarin, Coumadin; a Diuretic; Seizure medication—Primidone, Carbamazepine, Phenobarbital, Fosphenytoin, Phenytoin, Oxcarbazepine. The drug (Delayed-Release Capsule) is contraindicated in patients with known hypersensitivity to substituted benzimidazoles or to any ingredient of the drug formulation.
Esomeprazole (Nexium)
Esomeprazole is a proton pump inhibitor of gastric parietal cells used to control and treat stomach acid. The drug is available under generic name Esomeprazole and brand names Nexium® and many others worldwide.
The drug is marketed in by Astra Zeneca Lp. and STAT Rx USA Ltd. Esomeprazole is generally used to treat symptoms of gastroesophageal reflux disease (GERD), peptic ulcer disease, dyspepsia, and Zollinger-Ellison syndrome (condition in which stomach produces large amount of acid), and in combination with other drugs for treatment of peptic ulcer caused by consumption of nonsteroidal anti-inflammatory drugs (NSAIDs) or by a certain type of bacteria (H. pylori).
Esomeprazole chemically belongs to the class of organic compounds which are known as sulfinylbenzimidazoles chemically characterized as polycyclic aromatic compounds containing a sulfinyl group attached to benzimidazole moiety.
Esomeprazole is a synthetic pharmaceutical organic compound named as (S)-6-Methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1H- benzo[d]imidazole. Esomeprazole is the S-isomer of omeprazole, which is a racemic mixture of R- and S- forms.
The compound has molecular formula C17H19N3O3S and the molecular weight of 345.461 g/mol. The melting point of Esomeprazole is 155 °C. It is slightly soluble in water with a maximum solubility of 0.353mg/ml.
The medication is supplied as a delayed-release capsule or suspension for oral administration that means the drug is directly release in intestine rather than stomach to prevent its breakdown by stomach acids.
Esomeprazole capsule is available in two strengths (20 mg and 40 mg), and as a delayed-release oral suspension in varying strength (2.5 mg, 5 mg, 10 mg, 20 mg and 40 mg).
Besides Esomeprazole as the active component, other ingredients of capsule includes hypromellose, magnesium stearate, glyceryl monostearate 40-55, hydroxypropyl cellulose, methacrylic acid copolymer type C, polysorbate 80, sugar spheres, talc, and triethyl citrate.
The capsule is usually swallowed as a whole or open and mixed with water to take by mouth or emptied into a syringe for administration through a feeding tube. The drug is usually recommended once a day and at least 1 hour prior to meal.
The mode of action of Esomeprazole is similar to the working of other proton pump inhibitors and involves inhibition of the H+/K+-ATPase in the stomach or gastric parietal cells responsible for the production of the acid thereby interfering with the final step in the production of the acid, and decreasing the gastric acidity.
The prescribed dose of Esomeprazole varies depending upon the age and the diseased state. Pharmacokinetic studies suggest that after oral administration, Esomeprazole is rapidly absorbed and shows bioavailability ranging from 50 – 90 %.
It has been observed that following single 20 to 40 mg oral doses of Esomeprazole, peak plasma concentrations of 0.5-1.0 mg/l is achieved within 1–4 hours. Following absorption, the majority (97%) of the drug is bound to plasma proteins.
The drug is metabolized mainly by the hepatic system. The drug is eliminated mainly in urine as metabolites (about 80%) and the remaining 20% as feces. The average median half-life of Esomeprazole is 1 to 1.5 hours.
The average steady state volume of distribution of Esomeprazole is approximately 16 litres. The Esomeprazole is classified by US FDA pregnancy category: C. Esomeprazole has received its official approval from US Food and Drug Administration (FDA) in February 2001.
The drug received official approval from FDA for the treatment of heartburn and other symptoms associated with gastroesophageal reflux disease (GERD), healing of erosive esophagitis, duodenal /peptidic ulcer disease caused by Helicobacter pylori infection in combination with amoxicillin and clarithromycin, gastric ulcers associated with use of nonsteroidal anti-inflammatory drugs (NSAIDs) and Zollinger-Ellison syndrome.
The common side effects of Esomeprazole include diarrhea, headache, dizziness, sneezing, joint pain, runny nose, sore throat etc. Esomeprazole is usually well tolerated at the dose limit of 10 mg/day.
However, in some individuals, Esomeprazole may cause the risk of serious side effects such as severe liver damage when administrated with statins or may cause muscles damage when taken with fibrate or statins.
Some of the common signs that need to be addressed immediately to the health care provider comprise muscle pain/weakness/tenderness (particularly in case of unusual tiredness or fever), liver problems such as severe abdominal or stomach pain, yellowish eyes/skin, dark urine and persistent nausea/vomiting.
Symptoms of severe allergic reactions associated with the administration of Esomeprazole may include rashes, severe dizziness, itching/swelling, trouble in breathing etc.
Esomeprazole may show interaction with some of the drugs which include Antifungals (such as Voriconazole, Ketoconazole, Itraconazole), Benzodiazepines (Diazepam), Calcium salts, Cilostazol, Clarithromycin, Clopidogrel, Clozapine, Nilotinib, Dasatinib, Erlotinib, Digoxin, Fluvoxamine, Iron salts (such as ferrous sulfate), Methotrexate, Mycophenolate, Protease inhibitors (like Atazanavir, Indinavir, Nelfinavir, Saquinavir), Rifampin: Rilpivirine, Tacrolimus, Tolterodine, Warfarin.
Esomeprazole is contraindicated in patients with known hypersensitivity to proton pump inhibitors. Hypersensitivity reactions known to be associated with the Esomeprazole include angioedema and anaphylactic shock.
Omeprazole vs Esomeprazole: Where the difference lies
- Omeprazole is a pro-drug which is converted in the parietal cells of the stomach to the active drug in contrast to the Esomeprazole which is the active drug.
- Omeprazole is an impure racemic mixture with R- and S- isomers. The sulfur group provides a chiral center resulting in two identical and equally effective (at the cellular level in the stomach) molecules with different 3D structure. In contrast, Esomeprazole lacks any distinct chiral isomer and is the S-isomer or basically a purified component of Omeprazole.
- Esomeprazole has greater activity than Omeprazole (racaemic mixture) due to the significantly lower activity of the R-isomer thereby bringing about the same degree of acid suppression with lower doses in comparison to Omeprazole.
- The bioavailability of Omeprazole and Esomeprazole differs from each other. Bioavailability of Omeprazole ranges from 30-76% and that of Esomeprazole ranges from 50 – 90 %.
- Omeprazole is cheaper than Esomeprazole and hence a better choice. Esomeprazole costs about 4-8 times than that of Omeprazole.
- After absorption approximately 95% of Omeprazole is bound to blood plasma in contrast to 97% in case of Esomeprazole.
- The prescription Omeprazole is available in the form of delayed release capsules and packets of delayed release granules. However, the prescription Esomeprazole is available only as delayed release capsule.
- Omeprazole is associated with adverse effects including nausea, abdominal pain, diarrhea, dizziness, etc. However, Esomeprazole is associated with an extended array of side effects that additionally include loss of appetite, constipation and dry mouth with more severe effects such as allergic reactions, dark urine, paresthesia, severe abdominal pain, etc.
