Contents
- What is Diphenhydramine (Benadryl)
- Diphenhydramine generic name brand name
- What is the source of the drug (natural or synthetic)
- What is diphenhydramine prescribed for
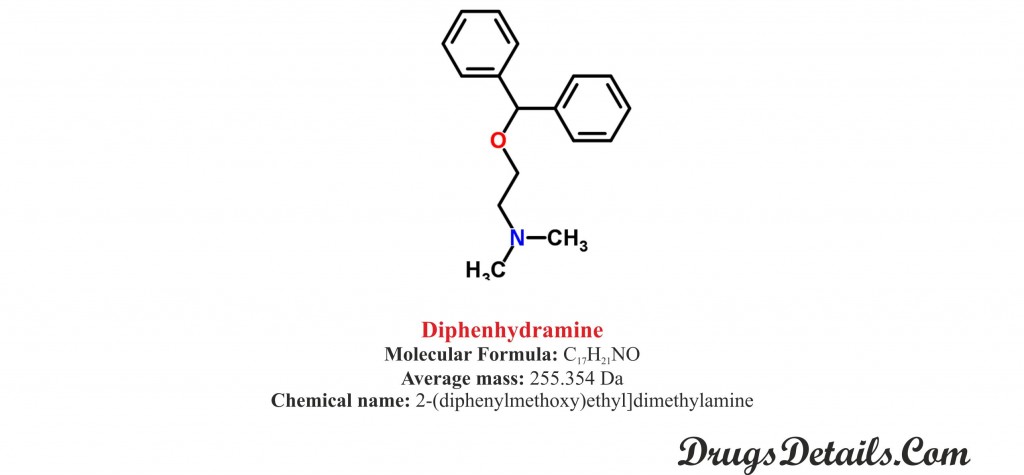
- Diphenhydramine chemical structure
- Chemical information of the drug
- Diphenhydramine strengths
- How diphenhydramine works
- Recommended dose of diphenhydramine
- When should I discontinue, withhold or modify the dose of Diphenhydramine
- What are the pharmacokinetic properties of the drug Diphenhydramine
- Which pregnancy category (A; B; C; D; X) has been assigned to Diphenhydramine
- How to use diphenhydramine
- Storage of diphenhydramine
- How to dispose of diphenhydramine
- Does Diphenhydramine has approval from government / FDA /or any other related agencies
- Other uses of the drug
- What special dietary precautions should I follow
- What special precautions should I follow/ what should I avoid while using Diphenhydramine
- Diphenhydramine side effects
- Overdose of diphenhydramine
- What should I do in case of missed a dose
- Diphenhydramine drug interactions
- Does Diphenhydramine have any interaction with diseases
- Where can I get more information
- Clinical research and current scenario of the drug
- References from chemical, biological and toxicological databases
What is Diphenhydramine (Benadryl)
- Diphenhydramine is a first generation antihistamine (anti-allergic agent) that is used to treat the symptoms of allergies.
Diphenhydramine generic name brand name
- The drug is available under generic name Diphenhydramine and various brand names such as Benadryl ®, Aler-Dryl®, Allergia-C, Allermax, Banophen, Unisom and Sominex.
- The drug was originally synthesized by George Rieveschl in 1943 and made publically in 1946.
- Diphenhydramine is manufactured and marketed under trade name Benadryl by McNeil Laboratories, a division of Johnson & Johnson.
What is the source of the drug (natural or synthetic)
- Diphenhydramine is a synthetic man-made histamine H1 antagonistic (antihistamine) pharmaceutical drug.
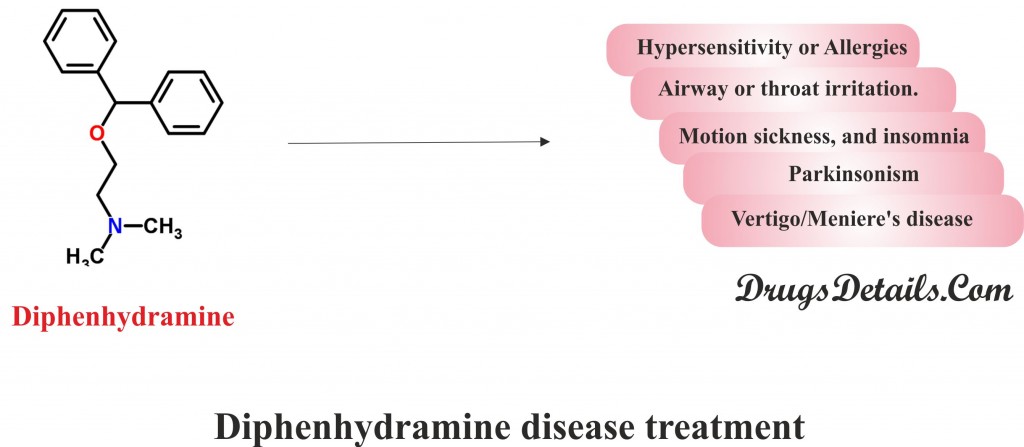
What is diphenhydramine prescribed for
- Diphenhydramine is an antihistamine agent that has antitussive, anticholinergic, antiemetic, and sedative properties and therefore used in the treatment of allergic symptoms.
- The drug is frequently used to relieve from allergic reactions such as itching, hives, skin rashes, sneezing, watery eyes, runny nose and other common symptoms caused by allergens, hay fever and common cold.
- Diphenhydramine is also prescribed for the treatment of cough caused by airway or throat irritation.
- Diphenhydramine is also used to relieve the motion sickness, and insomnia (a sleep disorder that is characterized by sleeplessness).
- Diphenhydramine is also used to treat drug-induced Parkinsonism (movement disorders where people have difficulty with movement) and other Extrapyramidal symptoms.
- Diphenhydramine is also used for management of Vertigo/Meniere’s disease, and insect bite.
Diphenhydramine chemical structure
Pharmacophore structure: Information about the chemical structure of the drug.
Diphenhydramine chemically belongs to the class of organic compounds which are known as Diphenylmethanes characterized by a diphenylmethane moiety. Diphenylmethane moiety consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. The detailed chemical classification of Diphenhydramine is described below:
| Kingdom | Organic compounds |
| Super Class | Benzenoids |
| Class | Benzene and substituted derivatives |
| Sub Class | Diphenylmethanes |
| Direct Parent | Diphenylmethanes |
Chemical information of the drug
- It is a synthetic pharmaceutical aromatic homomonocyclic compound with a molecular formula C17H21
- The molecular weight of Diphenhydramine is 354 Da.
- Chemically, Diphenhydramine is known as 2-(diphenylmethoxy)ethyl] dimethylamine.
- Diphenhydramine is white, odourless crystalline powder and has water solubility of 3060 mg/L at 37°
- Diphenhydramine is freely soluble in methanol and ethanol (~500 mg/ml) and less soluble in DMSO (~58 mg/ml).
- The melting point of Diphenhydramine is 161-162°
- Diphenhydramine is available as a monohydrochloride salt.
Diphenhydramine strengths
- Diphenhydramine is available in tablet, capsule and liquid form for oral administration, in parenteral form for intravenous or intramuscular use as well as in the form of topical cream.
- Diphenhydramine tablets are available in different dose strength of 12.5 mg and 25 mg.
- Tablets of 12.5 mg are purple in color, round in shape and debossed with “BENADRYL” over “12.5” while 25 mg tablets are pink in color, capsule shaped embossed with “B” on one side and debossed with “WL 25” on another side corresponding to the dose of the drug.
- Diphenhydramine tablets contain Diphenhydramine HCl as active ingredient and microcrystalline cellulose, hypromellose, polyethylene glycol, croscarrmellose sodium, carnauba wax, dibasic calcium phosphate, magnesium stearate, polysorbate 80, titanium dioxide and D&C Red 27 Aluminum Lake as inactive ingredients.
- Diphenhydramine capsules are available in different strength of 25 mg and 50 mg.
- Capsules are white in color with pink cap and consist of Diphenhydramine HCl as an active ingredient and lactose and talc as inactive ingredients.
- Capsule shell contains gelatine, silicon dioxide, sodium lauryl sulfate, titanium dioxide, FD&C Blue No. 1 and FD&C Red No. 3.
- Diphenhydramine parenteral injections are available in different strength of 10 mg/ml and 50 mg/ml.
- In addition, multi-dose vials contain 0.1 mg/ml germicidal agent benzethonium chloride.
- Diphenhydramine cream contains 2% Diphenhydramine hydrochloride (w/w).
- Inactive ingredients in Diphenhydramine cream are paraben, polyethylene glycol, cetyl alcohol, propylene glycol and water.
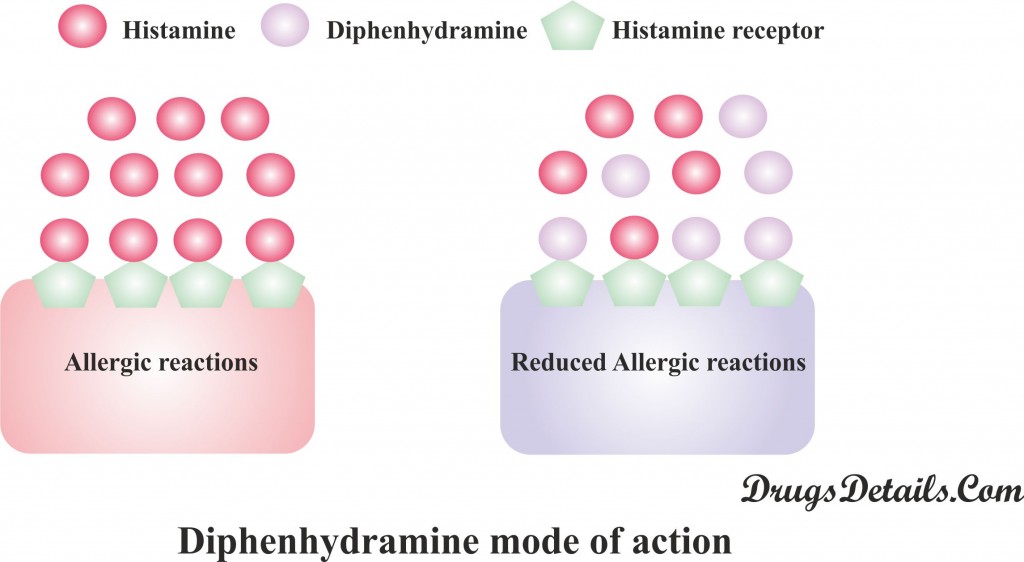
How diphenhydramine works
- Diphenhydramine is an antihistamine compound that belongs to the class ethanolamine.
- Principally it acts through inhibition of histamine (nitrogenous organic compound that acts as a neurotransmitter and concerned with local immune response), a key substance that causes allergic symptoms.
- Mode of action of Diphenhydramine is different from other antihistamines (like cromolyn and nedocromil which prevents the release of histamine). Diphenhydramine competes with free histamine for binding at HA-receptor site.
- Binding of Diphenhydramine on HA-receptor site provoke the effect of histamine on HA-receptors.
- This leads to a decline of the negative symptoms brought on by histamine HA-receptor binding.
Recommended dose of diphenhydramine
The prescribed dose of Diphenhydramine varies depending upon the age and diseased state of the patient.
Adult dose
- Cold symptoms: 25 to 50 mg in every 4 to 6 hour in a day orally (maximum up to 300 mg in a day).
- Cough: 25 mg in every 4 hour orally (maximum 150 mg in a day).
- Insomnia: 25-50 mg once a day usually at bedtime.
- Motion sickness
- Intravenous or intramuscular: 10-50 mg in a day, dose may increase up to 100 mg if patient needs (maximum 400 mg in a day).
- Oral: 25-50 mg three or four times in a day.
- Parkinsonian syndrome
- Intravenous or intramuscular: 10-50 mg in a day.
- Oral: 25-50 mg three or four times in a day.
- Pruritus: 25 to 50 mg in every 4 to 6 hour in a day orally (maximum up to 300 mg in a day).
- Urticaria: 25 to 50 mg in every 4 to 6 hour in a day orally (maximum up to 300 mg in a day).
Paediatric dose
- Allergic rhinitis
- 2 to 6 years:25 mg in every 4 to 6 hour in a day orally (maximum up to 37.5 mg daily).
- 6 to 12 years: 5-25 mg in every 4 to 6 hour in a day orally (maximum up to 150 mg daily).
- More than 12 years: 25-50 mg in every 4 to 6 hour in a day orally (maximum up to 300 mg daily).
- Cold symptoms
- 2 to 6 years:25 mg in every 4 to 6 hour in a day orally (maximum up to 37.5 mg daily).
- 6 to 12 years:5-25 mg in every 4 to 6 hour in a day orally (maximum up to 150 mg daily).
- More than 12 years: 25-50 mg in every 4 to 6 hour in a day orally (maximum up to 300 mg daily).
- Cough
- 2 to 6 years:25 mg in every 4 hour in a day orally (maximum up to 37.5 mg daily).
- 6 to 12 years:5 mg in every 4 hour in a day orally (maximum up to 75 mg daily).
- More than 12 years: 25 mg in every 4 hour in a day orally (maximum up to 150 mg daily).
- Insomnia
- 12 or more than 12 years: 25-50 mg once in a day orally at bedtime.
- Motion sickness
- 2 to 6 years:25 mg in every 4 to 6 hour in a day orally (maximum up to 37.5 mg daily).
- 6 to 12 years:5-25 mg in every 4 to 6 hour in a day orally (maximum up to 150 mg daily).
- More than 12 years: 25-50 mg in every 4 to 6 hour in a day orally (maximum up to 300 mg daily).
- Parkinsonian syndrome
- Intravenous or intramuscular: 1-2 mg/kg in a day.
When should I discontinue, withhold or modify the dose of Diphenhydramine
- The usual dosing of the drug may vary depending upon the efficiency and side effects of the drug in a particular individual.
- Do not use the medicine if you are hypersensitive or allergic to any of the ingredients of Diphenhydramine.
- Before taking Diphenhydramine, tell your doctor about your medical history preferentially if you have heart disease, low blood pressure, liver disease or kidney disease.
- Diphenhydramine is not used in patients with glaucoma, asthma, bronchitis, emphysema, chronic obstructive pulmonary disorder or other breathing problem.
- Take advice from your medical health provider if you have thyroid disorder and phenylketonuria.
- Diphenhydramine should not be used with the medications of anxiety, depression, muscle relaxant and narcotic medication for pain.
- Diphenhydramine is contraindicated with sleeping pills, sedatives and tranquilizers.
- Diphenhydramine may be contraindicated if you are taking monoamine oxidase inhibitors (MAOIs) such as Isocarboxazid, Linezolid, Phenelzine, Selegiline and Tranylcypromine.
- Diphenhydramine may also be contraindicated if you are taking inhibitor of CYP 2D6 such as Fluoxentine.
What are the pharmacokinetic properties of the drug Diphenhydramine
- Pharmacokinetic studies suggested that after oral administration Diphenhydramine is well absorbed in gastrointestinal tract and has a bio-availability of approximately 40-60%.
- Following absorption the majority (98%-99%) of the drug is bound to plasma proteins.
- It has been observed that following a single dose of 50 mg, maximum (or peak) plasma concentration (83ng/ml) is achieved in 120-180 minutes in the fasted state.
- The drug is mostly metabolized in liver primarily by cytochrome P450 2D6. Cytochrome P450 1A2, P450 2C19 and P450 2C9 may also be involved in the metabolism of
- Diphenhydramine is metabolised into nordiphenhydramine (active metabolite), dinordiphenhydramine, and diphenylmethoxyacetic acid through two successive demethylations and then oxidation.
- The average median half-life of Diphenhydramine ranges from 1 to 4
- Diphenhydramine is mainly excreted in urine as metabolites (94%) with some amount of uncharged drug (1.9%). Remaining 6% drug is excreted through feces in the form of metabolites.
- The average steady state volume of distribution of the Diphenhydramine is approximately 28 L/kg.
Which pregnancy category (A; B; C; D; X) has been assigned to Diphenhydramine
- The Diphenhydramine is classified by US FDA pregnancy category: B
- Due to lack of adequate and well-controlled studies the use of Diphenhydramine in pregnant women is contraindicated and recommended only when benefit justifies the risk.
- Laboratory animal studies have shown no adverse effect on the fetus.
- Studies support the excretion of Diphenhydramine into human milk. It may hold back the lactation. Therefore, breast-feeding is not recommended.
- Despite these facts, caution should be exercised when taking Diphenhydramine.
How to use diphenhydramine
- Diphenhydramine is available in tablet, capsule and liquid form for oral administration.
- Diphenhydramine is also available in parenteral form for intravenous use as well as in the form of topical cream.
- Diphenhydramine capsule should not be chewed, split or crushed. Whole tablet should be swallowed.
- Diphenhydramine oral liquid should be measured with measuring spoon provided with the medication.
- It is recommended to take the drug at almost the same time every day.
- Diphenhydramine is not recommended for use in patients under the age of 2 years.
- Diphenhydramine is also not safe for the patients who are older than 65 years. Diphenhydramine should be used with precaution in these patients.
- Follow the instructions carefully as directed on the prescription leaflet and take Diphenhydramine exactly as directed.
- Do not change the dose of the drug as prescribed by your doctor as the dosage is based on patient medical condition, treatment response and usage with other drugs.
Storage of diphenhydramine
- Diphenhydramine is stored at 25°C (77°F) and excursion permitted to 15-30°C (59-86°F).
- Diphenhydramine parenteral injection should be prevented from freezing.
- Store the medicine away from light and moisture.
- Medicine should not be stored in the bathroom.
- The drug should be kept away from children and pets.
How to dispose of diphenhydramine
- Throw away unused and opened, outdated or no longer used container.
- Also dispose the old medicine after the expiration date.
- Consult your pharmacist or local waste disposal company for proper disposal.
- Diphenhydramine was first approved in 1946 by U.S. Food and Drug Administration for the treatment of allergy and symptoms of cold and cough.
- Diphenhydramine injection was approved by U.S. FDA in March 1947.
- Preservative free Diphenhydramine injection was approved by U.S. FDA in December 1957.
Other uses of the drug
- Diphenhydramine is also used for the treatment of nausea that occurs in motion sickness.
- Diphenhydramine may also be used for other purposes not listed here. It is advisable to ask your doctor or pharmacist for more information.
What special dietary precautions should I follow
- Consult your doctor or pharmacist regarding the use of grapefruit products.
- Alcohol consumption can also enhance some side effects of the drug and thus, should be avoided.
What special precautions should I follow/ what should I avoid while using Diphenhydramine
- Consult with your doctor and pharmacist if you are taking any prescription and non-prescription medications or herbal products.
- Do not use other cough or cold medications that contain
- Avoid direct exposure to sunlight because Diphenhydramine medication may cause sensitivity to sunlight.
- Avoid using machinery requiring alertness or clear vision as well as car driving as the use of Diphenhydramine may make you drowsy.
- Do not use alcohol and alcoholic beverages; it may enhance the frequency of some side effects such as drowsiness.
- Consult your doctor in case of any query.
Diphenhydramine side effects
In addition to the associated benefits, Diphenhydramine also is accompanied with the side effects some of which are more common, others less common whereas some are more serious. It is always recommended to consult a doctor if you encounter any of the side effects.
Most common side effects caused by Diphenhydramine requiring medical attention are as follows:
- Constipation
- Muscle weakness
- Dry mouth, nose, and throat
- Headache
- Chest congestion
- Nervousness
- Dizziness
- Loss of appetite
- Drowsiness
- Nausea
- Vomiting
Diphenhydramine may cause some serious side effects which require immediate medical treatment and are as follows:
- Difficulty urinating
- Vision problems
- Painful urination
Besides these, Diphenhydramine may also be associated with some other side effects. These include:
- Ocular effects: blurred vision, eye dryness and diplopia.
- Genitourinary effects: dysuria and urinary retention.
- Cardiovascular effects: tachycardia, hypotension and palpitation.
- Hypersensitivity effects: rashes, photosensitivity, pruritus and eczema.
- Nervous system effects: dizziness, lip and tongue protrusion, depression, mental confusion, drowsiness, rigidity.
- Haematological effects: thrombocytopenia, agranulocytosis and haemolytic anaemia.
Diphenhydramine may also cause some other common or serious side effects which are not listed here. It is advisable to ask your doctor or pharmacist for more information.
Overdose of diphenhydramine
- If you overdose the drug contact with your doctor or pharmacist for symptomatic and supportive measures.
- Overdose of Diphenhydramine may cause circulatory collapse, respiratory and cardiac arrest, coma, anxiety, hyperactivity, dilated and sluggish pupils and fever.
What should I do in case of missed a dose
- Take a missed dose as soon as possible.
- To make up the missed dose, do not take extra medicine.
- Avoid taking the missed dose if it is time for your next dose.
Diphenhydramine drug interactions
Diphenhydramine can interact with a variety of medications (over 700 medications) that may result in decreasing or increasing the pharmacological effects of the drug.
These drugs can lead to either major or moderate drug interactions and require dosage adjustment. It is recommended to inform your doctor about all the prescription, non-prescription or herbal products you are taking before administrating Diphenhydramine and avoid any change or discontinuation of the drug without consulting the doctor.
- Significant Drug Interactions: The drugs that lead to significant drug interaction include the following
- Acetylcholinesterase inhibitor medications: Diphenhydramine when co-administered with anticholinesterase drugs used to treat Dementia and Alzheimer’s disease such as Tacrine (Cognex), Galantamine (Razadyne), Donepezil (Aricept), or Rivastigmine (Exelon) leads to lowering of the effects of both the drugs. Dosage adjustments and medical supervision is required to prevent the drug interactions.
- Drugs with anticholinergic effects: Diphenhydramine leads to increased side effects when administered with anticholinergic drugs used for the treatment of respiratory disease, gastrointestinal problems, and Parkinson’s disease. These side effects include dryness of nose, mouth and eyes; constipations; and less urine output, difficulty in passing urine. It is therefore recommended to inform your doctor about any of the following anticholinergic drugs you are taking:
- Benztropine (Cogentin)
- Clidinium (Librax)
- Atropine
- Clozapine (Clozaril)
- Belladonna (Donnatal, B&O Supprettes, Bellamine S)
- Darifenacin (Enablex)
- Haloperidol (Haldol)
- Glycopyrrolate (Robinul)
- Pramlintide (Symlin)
- Homatropine (Hycodan)
- Dicyclomine (Bentyl)
- Scopolamine (Transderm Scop)
- Hyoscyamine (Levsin)
- Tolterodine (Detrol)
- Solifenacin (Vesicare)
- Ipratropium (Atrovent)
- Oxybutynin (Ditropan, Oxytrol)
- Tiotropium (Spiriva)
- Drugs with drowsiness effects: It is generally recommended to consult the doctor if you are taking medications belonging to the category of drugs that cause drowsiness as they may result in lengthening and exaggeration of the side effects including confusion, dizziness, and drowsiness. These include:
- Sleep medications (e.g., zolpidem (Ambien) and others)
- Antipsychotic medications (e.g., Risperidone, Trazodone, Chlorpromazine, Amitriptyline)
- Muscle relaxants
- Tricyclic antidepressants (e.g., Amitriptyline)
- Anxiety medications like Benzodiazepines, including, Lorazepam (Ativan), Alprazolam (Xanax), Diazepam (Valium), Clonazepam (Klonopin)
- Anti-seizure drugs (e.g., Carbamazepine)
- Narcotic pain relievers (e.g., Codeine)
- Alcohol and CNS depressants like sedatives, tranquilizers
- Monoamine Oxidase Inhibitors (MAOIs): Co-administration of MAOIs such as Linezolid (Zyvox), Methylene Blue, Phenelzine (Nardil), Selegiline (Emsam, Eldepryl, Zelapar), Procarbazine (Matulane), Rasagiline (Azilect), Moclobemide (Manerix), Isocarboxazid (Marplan), Tranylcypromine (Parnate) with Diphenhydramine can intensify and extend local side effects including difficulty passing urine; dryness of mouth, eyes and nose; constipation; and drowsiness.
- Other Antihistamines: Concomitant use of Diphenhydramine and antihistamines like Dimenhydrinate (Dramamine) increases the side effects.
- Drugs for treating diabetes: Diphenhydramine when co-administered with drug like Pramlintide used for treating diabetes can result in increased nausea and constipation.
This list does not contain all the possible drugs that can interact with Diphenhydramine. It is always advisable to discuss about any medication that you are taking before starting Diphenhydramine therapy.
Does Diphenhydramine have any interaction with diseases
Before you take Diphenhydramine, it is necessary to discuss any medical condition or allergies you have or any other significant medical fact. It has been observed that following medical conditions (disease) may interact with Diphenhydramine:
- Renal/Liver disease: Patients of renal disorder or liver dysfunction show increased accumulation of Diphenhydramine and its metabolites and hence are more exposed to the adverse effects of the drug. It is therefore recommended to initiate therapy in such patients with lower dosage.
- Chronic Obstructive Pulmonary Disease or Asthma: The anticholinergic effect of the Diphenhydramine may bring about obstruction in the respiratory tract by decreasing the volume of bronchial secretions and increasing its viscosity. It is therefore recommended to use the drug cautiously in patients with asthma or chronic obstructive pulmonary disease.
- Gastrointestinal Obstruction, Urinary Retention, Glaucoma/Intraocular Hypertension: Diphenhydramine is associated with anticholinergic activity and hence should be given cautiously to the patients with various disorders such as urinary obstruction or retention; angle-closure glaucoma, uncontrolled primary open-angle glaucoma, or untreated intraocular hypertension; and gastrointestinal obstructive disorders. The anticholinergic activity of the drug may result in more deterioration of the preexisting conditions of such patients. Elderly patients are more prone to the effect.
- Skin conditions: The drug Diphenhydramine in topical form (cream) should not be used in case of measles, chickenpox, or on extensive areas of the skin. Consult your doctor immediately in case the condition deteriorates and when the drug is continued for more than a week. It is advisable to avoid the use of medications containing Diphenhydramine while using the cream.
- Cardiovascular disease, Hyperthyroidism, Hypotension: The drug Diphenhydramine may result in cardiovascular adverse effects including ECG changes, Arrhythmias, Palpitation, Tachycardia, Hypertension, and Hypotension owing to its anticholinergic and local anesthetic activity. These effects are however rare and encountered only in case of overdose.
Where can I get more information
Your pharmacist or health care provider can provide more information about Diphenhydramine.
Clinical research and current scenario of the drug
- Clinical studies related to combination therapy of Trimethobenzamide and Diphenhydramine (TMB/DPH) provide evidences of effective relief in a high proportion of migraineurs.
- Studies indicate the efficacy and tolerability of novel combination of Naproxen sodium (NS) at a dose of 440 mg and Diphenhydramine (DPH) at a dose of 50 mg in subjects with postoperative dental pain along with transient insomnia in improving sleep and pain.
- Reproduction studies involving Diphenhydramine hydrochloride in rats and rabbits models at doses up to 5 times the human dose showed no evidence of impaired fertility or fetus toxicity.
- Clinical findings suggest Valerian-hops combination and Diphenhydramine as useful adjuncts in the treatment of mild insomnia.
- Studies indicate the efficacy of Diphenhydramine (DPH) as a sedative, antiallergic or antiemetic substance.
- Randomized, double-blind, and placebo controlled study indicate the efficacy of Diphenhydramine at varying doses of 25 mg and 50 mg in treating seasonal allergic rhinitis.
- Laboratory studies indicate a decrement in alertness, reaction time, learning ability, impairment in concentration, time estimation, attention and memory within the first 2-3 hours after drug administration.
- Simultaneous use of Diazepam and Diphenhydramine causes significant decrease in performance at 2 hours, and to some degree up to 4 hours.
References from chemical, biological and toxicological databases
- Diphenhydramine: MedlinePlus Drug Information. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a682539.htmlDrugBank
- Diphenhydramine (DB01075). drugbank.ca/drugs/DB01075
- Diphenhydramine | C17H21NO | ChemSpider. chemspider.com/Chemical-Structure.2989.html
- Diphenhydramine versus nonsedating antihistamines for acute allergic reactions: a literature review. http://www.ncbi.nlm.nih.gov/pubmed/17883909
- Efficacy of diphenhydramine against cough in humans: a review. http://www.ncbi.nlm.nih.gov/pubmed/17486423
- A clinical trial of trimethobenzamide/diphenhydramine versus sumatriptan for acute migraines. http://www.ncbi.nlm.nih.gov/pubmed/16732839
- Inhibition of cough reflex sensitivity bydiphenhydramine during acute viral respiratory tract infection. http://www.ncbi.nlm.nih.gov/pubmed/25673148
- Doxepin and diphenhydramineincreased non-rapid eye movement sleep through blockade of histamine H1 receptors. http://www.ncbi.nlm.nih.gov/pubmed/25498564
- Use of topical lidocaine, diphenhydraminehydrochloride, nystatin, and gabapentin swish in treatment for post-radiation neuropathy and oral mucositis. http://www.ncbi.nlm.nih.gov/pubmed/24850125
- The effect of diphenhydramineon sleep in pediatric burn patients: a secondary analysis. http://www.ncbi.nlm.nih.gov/pubmed/24722664
“Can you give Benadryl and ibuprofen at the same time?”
“Can you take two different antihistamines at the same time?”
Carvedilol 6.25 mg“
