Contents
- What is levetiracetam?
- What is levetiracetam used for?
- Levetiracetam drug class, molecular formula, weight
- How levetiracetam works in the body?
- Levetiracetam side effects
- Levetiracetam dosage
- Who should avoid levetiracetam? Warnings during levetiracetam use
- Is it safe to use levetiracetam during pregnancy?
- Is it safe to use levetiracetam during breastfeeding?
- Can levetiractam (Keppra) cause behavioral problems?
- Can levetiractam (Keppra) cause sleepiness and fatigue?
- Can levetiracetam cause angioedema or anaphylaxis?
- Can levetiracetam cause blood-test abnormalities?
- Can levetiracetam cause increased blood pressure?
- Levetiracetam overdose
- Can levetiracetam cause suicide thinking?
- Can levetiracetam cause coodridnation problems?
- Can patients with renal impairment take levotiaracetam safely?
- Levetiracetam absorption, distribution, metabolism and elimination
- Can levetiracetam be used in combination with other antiepileptic drugs?
- Can levetiracetam be taken together with alcohol?
- Can I take levetiracetam and Darvon together?
- Can I take levetiracetam and buprenorphin together?
- Can I take levetiracetam and Advil together?
- Can I take levetiracetam and methocarbamol together?
- Can I take levetiracetam and Benadryl together?
- Can I take levetiracetam and tramadol together?
- Can I take levetiracetam and Evening Primrose Oil together?
- Can I take levetiracetam and warfarin together?
- Can I take levetiracetam and oral contraceptives together?
- Can I take levetiracetam and Digoxin together?
- Can I take levetiracetam and Xanax together?
- Proper storage of levetiracetam
What is levetiracetam?
Levetiracetam is a Generic name for an anticonvulsant drug that is used to treat epilepsy. It is a prescription drug used to treat different types of seizures including: partial-onset seizures, primary generalized tonic-clonic seizures and myoclonic seizures. Levetiracetam is also used in combination with other antiepileptic drugs.
This drug selectively prevents hypersynchronization that may lead to epileptic attack by burst firing and further propagation of seizure activity. Levetiracetam is most commonly sold under the Brand name Keppra. Other common brand names are Spritam and Elepsia XR. Levetiracetam is available as an immediate-release tablet, an extended-release tablet, and as a liquid solution.
It also comes as an injectable solution. It is available in doses of 250 mg, 500 mg, 750 mg, 1000 mg and 1500 mg for oral route and in doses of 5 mg/ml, 10 mg/ml and 15mg/ml as a solution for injection or in 100 mg/ml as a concentrate for injection solution. FDA first approved levetiracetam in 1999 under the Brand name Keppra which is originally manufactured by UCB Group of companies.
What is levetiracetam used for?
Levetiracetam is most commonly prescribed to treat partial onset seizures in adults and children older than 4 years of age. It is also prescribed to treat tonic-clonic seizures in adults and children who are at least 6 years old, and myoclonic seizures in adults and children older than 12 years of age.
It is used as a monotherapy for the treatment of partial seizures, or in combination with other antiepileptic drugs for partial, myoclonic, and tonic-clonic seizures. Studies showed that this drug can reduce partial (focal) seizures by 50% or more when used in combination. FDA approved (labeled) indications for levetiracetam are:
- Partial Onset Seizures
- Status Epilepticus
- Myoclonic seizures
- Primary generalized tonic-clonic seizre
Levetiracetam is sometimes used off-label for following indications:
- Subarachnoid hemorrhages
- Tourette syndrome
- Anxiety disorder
- Alzheimer’s disease
- Neuropathic pain
- Essential tremors
- Autism
Levetiracetam drug class, molecular formula, weight
Levetiracetam belongs to the class of organic compounds known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately attached to the carboxylate group (alpha carbon), or a derivative thereof. Chemically it is the S-enantiomer of etiracetam.
Levetiracetam molecule structure:
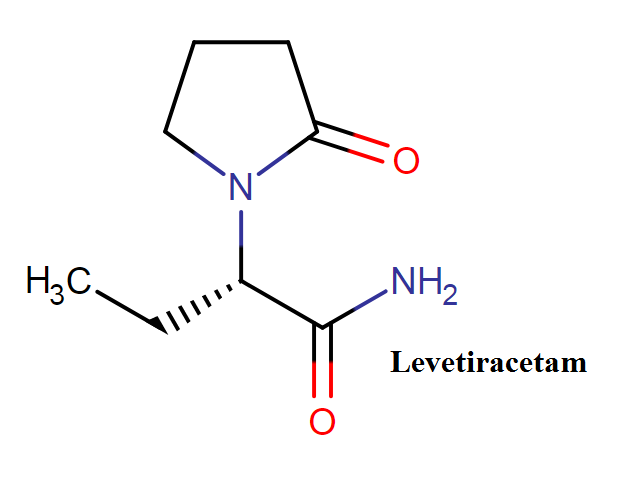
Levetiracetam molecular formula: C8H14N2O2
Levetiracetam molecular weight: 170.209 g/mol
Levetiracetam 3D formula:
How levetiracetam works in the body?
The precise mechanism of action by which levetiracetam exhibit its antiepileptic effect is unknown. Animal models studies revealed that levetiracetam did not inhibit single seizures attack when it was induced by maximal stimulation with electrical current or with chemoconvulsants. But, protection was found against secondarily generalized actions from focal seizures which were induced by two chemoconvulsants: pilocarpine and kainic acid.
These 2 substances can induce seizures by mimicking partial seizures with secondary generalization. Levetiracetam also displayed inhibitory activity in rat model. Pharmacologically, it is suggested that Levetiracetam can stimulate synaptic vesicle protein 2A (SV2A), with the inhibioton of neurotransmitter release.
Levetiracetam side effects
Levetiracetam may cause following side effects with following incidence:
- Asthenia (11-15%)
- Headache (14-19%)
- Infection (11-15%)
- Increased blood pressure (17% in children < 4 years)
- Somnolence (11-15%)
- Drowsiness (2-23%)
- Fatigue (10-11%)
- Anorexia (3-13%)
- Weakness (9-15%)
- Nasopharyngitis (7-15%)
- Cough (2-11%)
- Viral infection (2%)
- Asthma (2%)
- Dizziness (5-9%)
- Nervousness (2-10%)
- Amnesia (2%)
- Anxiety (2-3%)
- Ataxia (3%)
- Depression (2-5%)
- Hostility (10%)
- Paresthesia (2%)
- Sinusitis (2%)
- Diplopia (2%)
- Amblyopia (2%)
- Conjunctivitis (2-3%)
- Albuminuria (4%)
Rare side effects (<1%) that can be caused with levetiracetam use are following:
- Abnormal hepatic function tests
- Dyskinesia
- Eczema
- Neutropenia
- Decreased hematocrit
- Leukopenia
- Suicidal tendencies
- Hepatitis
- Pancreatitis
- Bone marrow suppression
- Epidermal necrolysis
Levetiracetam dosage
Partial onset seizures dosing
Adults (16+): Initiate treatment should be started with a daily dose of 1000 mg per day, given 2 times a day (500 mg twice daily). Additional dosing increments may be given (1000 mg/day additional every 2 weeks) to a maximum recommended daily dose of 3000 mg. There are no data that doses larger than 3000 mg/day may have additional benefit.
Pediatric patients (4 to 16 years): Initiate treatment should be started at daily dose of 20 mg/kg with 10 mg/kg twice daily. The daily dose should be increased every 2 weeks by increments of 20 mg/kg to the recommended daily dose of 60 mg/kg (divided in 2 doses of 30 mg/kg and taken twice daily). If a pediatric patient cannot tolerate the daily dose of 60 mg/kg, it can be reduced. The maximum daily dose is 3000 mg/day.
Pediatric patients (6 months to 4 years): Initiate treatment should be started with a daily dose of 20 mg/kg divided in 2 doses per day (10 mg/kg twice daily). In every 2 weeks the daily dose should be increased by an increment of 20 mg/kg to the recommended daily dose of 50 mg/kg divided in 2 doses (25 mg/kg twice daily). If a kid cannot tolerate a daily dose of 50 mg/kg, the daily dose can be reduced.
Pediatric patients (1 month to 6 months): Initiate treatment should be started with a daily dose of 14 mg/kg divided in 2 doses (7 mg/kg twice daily). The daily dose should be increased every 2 weeks by increments of 14 mg/kg to the recommended daily dose of 42 mg/kg divided in 2 doses (21 mg/kg twice daily). The effectiveness of lower doses has not been studied.
Myoclonic seizures dosing
Initiate treatment should be started with a dose of 1000 mg per day, divided in 2 daily doses (500 mg twice daily). On every 2 weeks dose should be increased by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg. The effectiveness of the lower doses than 3000 mg/day has not been studied.
Primary Generalized Tonic-Clonic Seizures dosing
Adults (16+): Initiate treatment should be started with a dose of 1000 mg per day, divided in 2 doses (500 mg twice daily). On every 2 weeks dose should be incresed by 1000 mg/day to the recommended daily dose of 3000 mg. The effectiveness of the lower doses than 3000 mg/day has not been adequately studied.
Pediatric patients (6 to 16 years): Initiate treatment should be started with a daily dose of 20 mg/kg divided in 2 doses (10 mg/kg twice daily). On every 2 weeks dose should be incresed by increments of 20 mg/kg (10 mg/kg twice daily) to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). The effectiveness of the lower doses than 60 mg/kg/day has not been adequately studied.
Who should avoid levetiracetam? Warnings during levetiracetam use
- Patients with renal issues. Levetiracetam is predominantly removed from the body via kidneys. In patients with lower kidney function, more amounts of the drug will stay in your body for a longer time. This may put such patients at higher risk for increased side effects.
- Patients with depression or mood problems. Levetiractam may worsen symptoms of already existing depression, mood problems, or may initiate suicidal thoughts or behavior. Patients should talk with their doctor whether this drug is safe for you to take.
- Pregnant women. This drug should be avoided during pregnancy. Talk to your doctor if you are pregnant or plan to become pregnant while using this drug.
- Women who are breastfeeding. This drug may pass into breast milk and may cause side effects in a child who is breast-fed. Avoid this drug use during breastfeeding
- This drug should not be used for the treatment of partial onset seizures in children younger than 1 month, generalized tonic-clonic seizures in children younger than 6 years and myoclonic seizures in children younger than 12 years. Increased blood pressure has been described in children taking levetiracetam between one month and 4 years old of age.
- Patient sensitive to this drug. Patients who are allergic to this drug should not take it. Taking it again could be fatal.
- Never abrupt the therapy immediately on your own. Never stop taking levetiracetam on your own without first speaking to a doctor. Abrupt discontinuation of treatment may lead to non-stop seizures. It’s also important that you take the exact dose prescribed.
Is it safe to use levetiracetam during pregnancy?
Levetiracetam is classified in a category C by the FDA pregnancy list of drugs which means that research in animals has shown side effects to the fetus and that there haven’t been enough studies done in humans to be certain how the drug might affect the fetus.
Animal studies have shown evidence of an increased occurrence of fetal damage when the drug was used in therapeutic or larger doses for humans. Talk to your doctor if you are using this drug and if you become pregnant or planning to become pregnant. This drug should only be used in situations if the potential benefit justifies the potential risk to the fetus.
Is it safe to use levetiracetam during breastfeeding?
Levetiracetam may pass into breast milk and cause side effects, thus this drug should not be used during breastfeeding without recommendation of the doctor, and should be taken only if benefits outweighs the risk. Studies showed that doses of 3500 mg taken by the mother may produce low levels in milk and are not expected to cause any adverse effects in breastfed infants, especially if the infants are older than 2 months.
If therapy with this drug is required by the mother, it is not a reason to discontinue breastfeeding. However, the infant should be monitored for drowsiness, developmental milestones, adequate weight gain, especially exclusively breastfed infants and when combinations of anticonvulsants are used.
Can levetiractam (Keppra) cause behavioral problems?
Yes, levetiracetam may cause behavioral changes and psychosis, so patient taking this drug should be monitored for such symptoms. Children are usually more vulnerable to this side effect.
One clinical study showed that 13 % of adult patients and 38 % of pediatric experienced behavioral changes such as: aggression, anger, agitation, anxiety, depersonalization, depression, apathy, emotional lability, hyperkinesias, hostility, irritability, nervousness, neurosis, and personality disorder.
Other study found that: 1% of Keppra treated adult patients, 2% of Keppra-treated pediatric patients within 4 to 16 years of age, and 17% of Keppra-treated pediatric patients of 1 month to 4 years of age experienced psychotic symptoms.
Can levetiractam (Keppra) cause sleepiness and fatigue?
Levetiracetam may cause somnolence and fatigue. Patients using this drug should be advised not to drive or operate machinery until they have gained sufficient experience how levetiraceam influence their body. Sleepiness and fatigue occurred most commonly within the first 4 weeks of treatment. In general, the incidences of these events in the pediatric and adults are comparable.
Can levetiracetam cause angioedema or anaphylaxis?
In rare cases levetiracetam may cause serious allergy reactions such as anaphylaxis or angioedema when the first dose is administrated. Signs and symptoms may include: hypotension, respiratory distress, hives, rash, and swelling of the face, eye, tongue, lip, mouth, throat, and feet. In some cases with levetiracetam, reactions were life-threatening and required emergency treatment.
If a patient develops anaphylaxis or angioedema, levetiracetam should be discontinued and the patient should seek immediate medical attention. Never take this drug again if you ever have any type of allergy during its use.
Can levetiracetam cause blood-test abnormalities?
Levetiracetam may cause blood-test abnormalities. Hematologic abnormalities occurred in clinical studies included red blood cells count (RBC) decrease, but also decrease of hemoglobin, and hematocrit, and increases in eosinophil counts. Some studies also showed white blood cells count (WBC) and neutrophil counts decrease. Rare cases of agranulocytosis have been also reported.
Can levetiracetam cause increased blood pressure?
It has been showed that levetiracetam may increase diastolic blood pressure in children younger than 4 years of age. If this drug is used in children, blood pressure should be carefully monitored.
Levetiracetam overdose
Levetiracetam overdose can happen if it is taken in dose of more than 6000 mg per day. The most sign of levetiracetam overdose is excess drowsiness. Other symptoms that may occur are: somnolence, depressed level of consciousness, agitation, aggression, respiratory depression, and coma.
There is no specific antidote for levetiracetam overdose. Induced emesis and gastric lavage on time are always recommendable. General supportive care of the patient is indicated including observing vital signs and monitoring of the patient’s clinical status.
A Certified Poison Control Center should be contacted in case of levetiracetam overdose. Standard hemodialysis procedures showed significant and efficient clearance of levetiracetam of about 50% in 4 hours and should be considered in overdose cases.
Can levetiracetam cause suicide thinking?
Levetiracetam can increase the risk for provoking suicidal thoughts or behaviors. Patients on its therapy should be monitored for mood or behavioral changes, depression worsening and suicidal thoughts. If such symptoms are observed, a doctor should be notified immediately.
Can levetiracetam cause coodridnation problems?
There are reported cases of coordination issues in adults taking levetiracetam. The problems typically occurred in the first 4 weeks of treatment. Because of that, patients should be monitored cautiously for any type of coordination difficulty, especially if patient is 60+ years old.
Can patients with renal impairment take levotiaracetam safely?
Levetiracetam is predominately eliminated unchanged via kidneys. Patients with renal impairment have lower efficacy to excrete levotiracetam regularly and decreased excretion is in correlation with cratinine clearance. Therapy with levetiracetam should be administered carefully with reduced dosages in patients with impaired renal function. Dosage adjustments should be based on the degree of renal impairment.
Levetiracetam absorption, distribution, metabolism and elimination
Absorption: Levetiracetam is almost completely and rapidly absorbed after oral administration.
Distribution: Levetiracetam and its metabolites have a less than 10% bound to plasma proteins therefore there are no clinically significant interactions with other drugs which have high affinity for protein binding.
Metabolism: Levetiracetam has not extensive metabolism. The major metabolic pathway is hydrolysis of the acetamide group. This major metabolite is inactive in animal seizure models. Two other metabolites are products of the hydroxylation of pyrrolidine ring (2% of dose) and opening of the pyrrolidine ring 5 (1% of dose). There is no enantiomeric reaction of interconversion of levetiracetam or its major metabolite.
Elimination: Levetiracetam elimination half-life is 7 ± 1 hour. 66% of Levetiracetam administered dose is eliminated from the systemic circulation through the renal excretion.
Can levetiracetam be used in combination with other antiepileptic drugs?
Levetiracetam is often used in combination with other antiepileptic drugs for the treatment of partial, myoclonic, and tonic-clonic seizures. Studies showed that levetiracetam taken in doses of 3000 mg daily had no effect on the pharmacokinetic of phenytoin in patients with refractory epilepsy.
Pharmacokinetics of levetiracetam was also not affected by phenytoin. There were also no interactions when levetiracetam was used in combination with valproates, carbamazepine, gabapentin, phenobarbital, lamotrigine, phenytoin and primidone.
Can levetiracetam be taken together with alcohol?
Levetiracetam and alcohol should not be taken together. Alcohol may potentiate some levotiracetam side effects such as CNS depression and/or impairment of judgment, thinking, and psychomotor skills.
Can I take levetiracetam and Darvon together?
Levetiracetam should not be taken together with Darvon as serious interaction may occur. If these drugs are taken together the risk of emotional disturbances and suicidal ideation may be increased. Propoxyphene is an inhibitor of CYP450 2D6 and may increase the plasma concentrations of propoxyphene which is the active ingredient of Darvon.
Can I take levetiracetam and buprenorphin together?
Buprenorphin should not be used together with CNS depressants such as levetiracetam. Concomitant use of buprenorphine and levetiracetam is associated with increased risk of buprenorphine overdose, respiratory depression, coma, and death.
There are reported cases that have primarily occurred in the setting of buprenorphine long-term treatment for opiate addiction, and many, but not all cases, involved buprenorphine abuse or misuse including intravenous self-injection. The true mechanism of interaction is still unknown, but some studies suggest that CNS depressants such as levetiracetam may alter the usual ceiling effect on buprenorphine-induced respiratory depression.
Can I take levetiracetam and Advil together?
Since there are no important interactions between these 2 drugs, they can be safely taken together.
Can I take levetiracetam and methocarbamol together?
Using levetiracetam and methocarbamol together may increase the risk of causing side effects such as drowsiness, dizziness, confusion, and difficulty concentrating. Some people, especially the elderly, may also experience impairment in judgment, thinking and motor coordination.
Can I take levetiracetam and Benadryl together?
CNS and/or respiratory-depressant effects may be increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
Can I take levetiracetam and tramadol together?
Levetiracetam should not be taken with tramadol together. There is an increased risk of causing CNS side effects such as dizziness, drowsiness, confusion, and difficulty concentrating.
Can I take levetiracetam and Evening Primrose Oil together?
It has been suggested that evening primrose, which contains the gamma linolenic acid, which is omega-6 fatty acid may lower the seizure threshold and block the effects of anticonvulsants. However there are no relevant data regarding the effect of gamma linolenic acid on threshold for seizure attack are conflicting and limited.
Several animal studies showed no effect on threshold of the seizure or even neuroprotective and anticonvulsant effects of gamma linolenic acid. However, the possibility of an interaction should be considered if loss of seizure control occurs in patients using evening primrose oil.
Can I take levetiracetam and warfarin together?
Studies showed no changes prothrombin time when levetiracetam was used together with warfarin. There are no pharmacokinetic interactions between these two drugs. They can be taken together safely.
Can I take levetiracetam and oral contraceptives together?
Studies showed no influence on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and levonorgestrel, or of the progesterone or luteinizing hormone levels, thus the impairment of oral contraceptive efficacy is unlikely to be caused.
Can I take levetiracetam and Digoxin together?
Since studies showed no important interaction between these two drugs, they can be safely taken together.
Can I take levetiracetam and Xanax together?
Levetiracetam and Xanax should not be taken together. If they are used together they may increase the risk of causing side effects such as drowsiness, confusion, dizziness, and difficulty concentrating. Some people, especially the elderly, may also experience impairment in judgment, thinking and motor coordination.
Proper storage of levetiracetam
Store the product at room temperature away from light and moisture. Never store the drug in the bathroom. Keep all medications away from children and also pets. Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company for more details about how to safely discard your product.
“Oxybutynin (Oral Route): Brand Names, Uses, Side Effects, Interactions, Pictures“
