Contents
- What is Livedo Reticularis?
- What causes the discoloration of the skin in livedo reticularis?
- What are typical signs and symptoms of livedo reticularis?
- Are there any systemic issues related with livedo reticularis?
- What is livedoid vasculopahty?
- Livedo reticularis vs Livedo racemosa
- Livedo reticularis causes
- Link between Hematologic/hypercoagulable antiphospholipid syndrome and livedo reticularis
- Endocrine disorders and livedo reticularis
- Autoimmune diseases as a cause of livedo reticularis
- Hypercoaguabile and hyperviscosity states as causes of livedo reticularis
- Embolic diseases as a cause of livdo reticularis
- Amantadine as a cause of livedo reticularis
- Minocycline as a cause of livedo reticularis
- Infections as a cause of livedo reticularis
- Livedo reticularis diagnosis
- What is Cutis marmorata telangiectatica congenita?
- Livedo reticularis treatment
What is Livedo Reticularis?
Livedo reticularis (LR) is an ischemic dermopathy condition that can be described as a reticular or “netlike” violaceous mottling, surrounding a pallorous central part of the skin.
It is most commonly a sign of underlying condition but it could also be idiopathic without any certain cause. Although livedo reticularis is mostly localized on the lower extremities, the upper extremities can be also affected.
Typical physical signs are characterized by transiently acute or chronic blotchy, from reddish-blue to purple, net‑like cyanotic pattern of the skin.
Livedo reticularis is a manifestation of cutaneous disturbance of blood flow that may be caused by variety of physiologic and pathologic conditions. They may be benign for example in case of cutis marmorata of infancy, or serious, in the cases of conditions such as: vasculitis of lupus erythematous, cancers, and endocrine disorders.
Physiological livedo or cutis marmorata is commonly seen on the legs of infants and young women in cold weather that improves on rewarming.
What causes the discoloration of the skin in livedo reticularis?
The discoloration of the skin is usually caused by swelling of the venules due to obstruction of capillaries caused by small blood clots. The blood clots in these small blood vessels might be indirectly affected by a condition that increases the risk of forming serious blood clots.
Such conditions may include: hyperlipidemia, nutritional deficiencies, microvascular hematological or anemia states, hyper- and autoimmune diseases, and drugs or toxins.
What are typical signs and symptoms of livedo reticularis?
Typical skin manifestations of livedo reticularis are:
- A net-like, red-blue discoloration surrounding pale central areas of the skin
- The network may comprise regular unbroken circles or an irregular broken pattern
- It is predominately localized on legs, and trunk but it can be also found on arms and rarely on different body parts.
- LR is more pronounced in cold weather
- In physiological LR, patients is feeling well, but the signs may improve as the skin warms
- In pathological LR, the rash is persistent, irreversible with rewarming, and the patient may be feling unwell
- The term livedo racemosa (LCR) is associated with pathological findings and livedo and is sometimes used when livedo is more generalized with irregular-broken shape. While this pattern is commonly recognized in Sneddon’s syndrome, it can also be found in other causes including APS and the ANCA-positive vasculitides
Some of systemic findings that can be related with LR may be:
- Venous and artherial thrombosis has been observed in patients with LR
- Higher rates of morbidity during prengnancy also appears to be higher in women with systemic lupus disease and signs of livedo, even when they were negative for antiphospholipid antibodies
- Accelerated atherosclerosis may be provoked
What is livedoid vasculopahty?
Livedo reticularis may be also present as a cutaneous ulcer or livedoid vasculopathy and atrophy blanche may be a consequence. It usually affects the lower extremities. It is localized bilaterally and is more common in summer and hot climates. It may affect people of all ages groups but is more common in young women. The cause is painful papular rash that develops into an ulcer.
The ulcer gradually heals leaving a white scar or atrophie blanche that is frequently star-shaped. Livedoid vasculopathy was first originally described as a sign of vasculitis; though the definition is that it is a vaso-occlusive phenomenon with intradermal venules thrombosis.
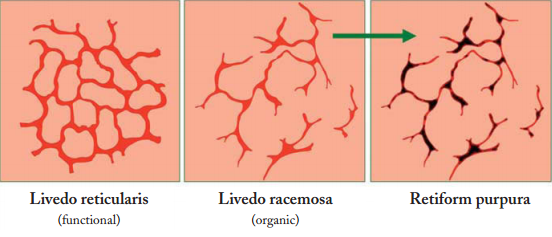
Livedo reticularis vs Livedo racemosa
Ehrmann in 1907 have been described two different patterns of livedo: The first one is physiological livedo reticularis (LR) and the second one is pathological livedo racemosa (LRC). The net-like rings in both types are caused by reduced blood flow and lowered oxygen perfusion at the skin peripheral segments.
The main differences between LR and LRC are that LR is a benign, primary physiologically disorder that most commonly affects young to middle-aged women. Discoloration is livid, conical and symmetric, reversible and uniform. On the other hand LRC is a secondary pathological and permanent disorder.
Differently from LR, in LRC has livid conical discoloration which is symmetric, irreversible and “broken”. Antiphospholipid antibody testing should be performed in all patients with LRC. In most cases, dermatologic presentation in patients with antiphospholipid syndrome (APS) is presenting in 25% of patients with primary APS and in 70% of patients with systemic lupus erythematosus related APS.
Physiological arteriolar vasospasm leads to reversible cutaneous discoloration in the case of livedo reticularis. Thus it usually occurs as a physiological response to cold exposure and in that case it is known as cutis marmorata, or the changes of the skin color may not be related to ambient temperature.
In the case of LRC – livedo racemosa, protracted arteriolar vasospasm, thrombosis, with or without hyperviscosity are pathological skin changes causes. Venodilatation of the venous plexus may be triggered by hypoxia or autonomic dysfunction.
An important role of the endothelial cells has also been suggested in patients with APS and LRC. It has been supposed that the interaction between APL antibodies with endothelial cells could induce LRC and may lead to increased production of procoagulant factors such as plasminogen activator inhibitor 1, tussue factor and endothelin. Increased tissue factor on endothelial cells induced by APL may be responsible, partly for hypercoagulability states.
Drug-induced LR may also happen. Amantadine has been reported to provoke LR as a result of catecholamine-induced arteriolar vasospasm.
Livedoid vasculopathy is a uncommon ulcerative subtype of livedo racemosa that may be caused due to fibrinolytic abnormalities and microcirculatory thrombosis.
Fibromyalgia and congenital hypogammaglobulinemia can be also associated with LR.
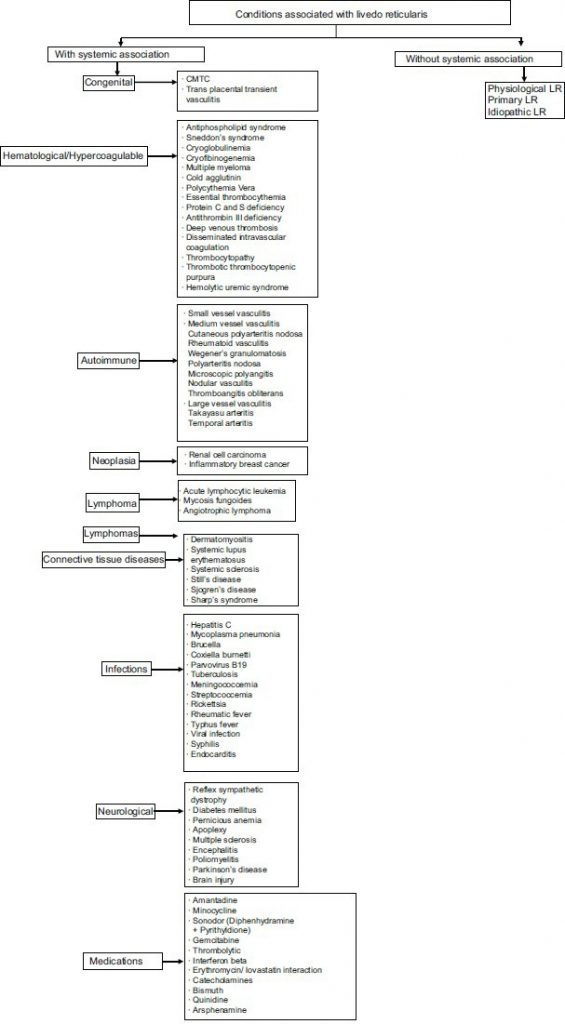
Livedo reticularis causes
Various conditions may cause the appearance of livedo reticularis:
- Cutis marmorata telangiectatica congenita – very rare condition
- Sneddon syndrome – genetic cause
- Idiopathic livedo reticularis – most common cause, unknown cause affecting mostly young women during the winter:
- Vasculitis
- Polyarteritis nodosa
- Systemic lupus erythematosus
- Dermatomyositis
- Rheumatoid arthritis
- Lymphoma
- Pancreatitis
- Chronic pancreatitis
- Tuberculosis
- Drug-related: Adderall, Amantadine, Bromocriptine, Beta IFN, Rasagiline, Methylphenidate Dextroamphetamine, Gefitinib
- Cryoglobulinaemia
- Antiphospholipid syndrome
- Hypercalcaemia
- Haematological disorders of polycythaemia rubra veraor thrombocytosis
- Infections (syphilis, tuberculosis, Lyme disease)
- Acute renal failure
- After cardiac catheterization
- Arteriosclerosis
- Homocystinuria
- Intra-arterial injection (especially in drug addicts)
- Ehlers-Danlos syndrome
- Pheochromocytoma
- Livedoid vasculopathy and its link with factor V Leiden mutation
- FILS syndrome
- Primary hyperoxaluria, oxalosis (oxalate vasculopathy)
- Cytomegalovirus infection
- Generalized livedo reticularis induced by silicone implants for soft tissue augmentation
- Down syndrome
- Idiopathic livedo reticularis with polyclonal IgM hypergammopathy
- CO2angiography (rare, reported case)
- Churg-Strauss syndrome
- Erythema nodosum
- Livedo vasculopathy linked with IgM
- Metastatic breast carcinoma (very rare)
- Renal cell carcinoma (rare)
- Buerger’s disease
- Graves hyperthyroidism
- Pernicious anaemia
- Moyamoya disease
- Related with the use of a midline catheter
- Familial primary cryofibrinogenemia
Retrieved from: Indian Dermatol Online J. 2015 Sep-Oct; 6(5): 315–321. (click for better resolution)
Link between Hematologic/hypercoagulable antiphospholipid syndrome and livedo reticularis
The strict definition of antiphospholipid syndrome is presence of lupus anticoagulant or anticardiolipin antibodies and either vascular thrombosis or specific complicationsduring pregnancy. There may be also different antiphospholipid antibodies related with the clinical features of the antiphospholipid syndrome, however they are not considered as specific in the diagnostic criteria.
The antiphospholipid syndrome may be primary which is not related with other diseases, and secondary which is related with other diseases, most commonly systemic with systemic lupus erythematous (SLE) or other auto-immune connective tissue disorders, drugs or malignancy induced.
Livedo reticularis has been shown to have a very high incidence of association with the antiphospholipid antibodies presence in the absence of SLE. One study has been shown that approxiamtely 40% of patients exhibit livedo reticularis as the first sign of their antiphospholipid syndrome.
According to this, a patient who has livedo reticularis should always be assessed for the presence of antiphospholipid antibodies. Likewise, consideration should be also made for evaluating patints with venous leg ulceration for the presence antiphospholipid antibodies, since it was proved that there is an association between these two.
About 1/3rd of patients with SLE may have antiphospholipid antibodies; however antiphospholipid syndrome might not be present in all patients. Moreover, livedo reticularis has been also proved to be a significant sign for neuropsychiatric lupus erythematosus development.
Patients with diagnosed antiphospholipid syndrome associated with SLE are more likely to have arthritis, thrombocytopenia, livedo reticularis and leukopenia than patients with primary antiphospholipid syndrome. On the other hand, patients with primary antiphospholipid syndrome are more likely to have asymptomatic avascular necrosis of the femoral head.
Endocrine disorders and livedo reticularis
Different studies have been demonstrated that hormonal may provoke livedo reticularis. Hypothyroidism can lead to livedo reticuaris and may be resolved with appropriate replacement therapy.
Pseudohypoparathyroidism with hypercalcemia has been also shown to cause extensive calcification and because of that may provoke ischemic skin signs such as livedo reticularis or skin infarction. Hypoparathyroidism may be also the cause. There are some findings suggesting that Cushing’s disease is also associated with livedo reticularis. Pellagra as a nutritional disorder is also associated with livedo.
Autoimmune diseases as a cause of livedo reticularis
SLE, systemic sclerosis, and Sjögren’s disease are most common autoimmine diseases linked with Livedo reticularis. Dermatomyositis and Still’s disease may also be the cause. If livedo reticularis occur in patients with rheumatoid arthritis rheumatoid vasculitis should be considered. In connective tissue disorders, vasospasm may lead to the development of livedo reticularis. Raynaud’s phenomenon is also common cause of livedo reticularis.
Hypercoaguabile and hyperviscosity states as causes of livedo reticularis
Coagulopathies associated with protein C and S or antithrombin III deficiencies are also related with livedo reticularis. Homocysteinemia and homocysteinuria are conditions commonly related with disruption in methionine metabolism that may lead hypercoagulability. Homocysteine levels may also be increased in blood and urine.
Polycythemia vera is also considered as a cause of LR. It is a condition in which flow is slow down through the cutaneous microvasculature. Increased viscosity of red blood cells in polycythemia vera may result in livedo reticularis. Moreover cutaneous findings may include erythromelalgia, ruddiness of the face, and rarely acral cyanosis.
Cryoglobulinemia caused hyperviscosity may results in livedo reticularis
Paraproteinemia and multiple myeloma increase in circulating immunoglobulins also leads to slower flow and may result in LR.
Embolic diseases as a cause of livdo reticularis
Cardiovascular procedures such as catheterization may provoke cholesterol emboli syndrome. This syndrome is characterized with limb pain, eosinophilia, renal failure and intact peripheral pulses. Following the development of livedo reticularis, patients may develop purpura, necrosis, ulceration, cyanosis, and gangrene.
Amantadine as a cause of livedo reticularis
Amantadine has been shown to be associated with livedo reticularis. The proposed mechanism may be the combination of catecholamine-induced vasospasm and the amantadine effects on NMDA receptors in the skin. The livedo usually resolve after amantadine withdrawal.
Minocycline as a cause of livedo reticularis
Long-term minocycline administration has been reported to induce a polyarteritis nodosa-like syndrome characterized with fever, arthritis, fatigue, livedo, and positive p-ANCA. Discontinuation of minocycline use usually results in resolution.
Infections as a cause of livedo reticularis
Different infections such as: syphilis, mycoplasma, tuberculosis, brucella, hepatitis B and C, streptococcemia, and different viruses.
Livedo reticularis diagnosis
Combination of detailed medical history, physical examination and laboratory testing can be very helpful in determinating livedo reticular diagnosis.
Livedo reticularis medical history
A detailed medical history is very important to recognize if there are any cases related with systemic disease. Following questions may be helpful for best anamnesis of LR:
Is there a present or a history of some autoimmune disease? Are there any symptoms to propose autoimmune disease? Livedo reticularis may be a manifestation of underlying autoimmune condition such as systemic lupus erythematosus (SLE), scleroderma, dermatomyositis, acute rheumatic fever, or rheumatoid arthritis.
Does the patient have Raynaud’s phenomenon? The presence of Raynaud’s phenomenon suggests a tendency towards vasospasm.
Is there a history of chronic renal disease? Livedo reticularis may be caused if calciphylaxis is present.
Is there a history of predisposition for hypercoagulable states? Family or personal history of thromboembolic conditions or early stroke should be evaluated. Malignancy should be also evaluated since it may cause hypercoagulable state.
Does patient have any bacterial or viral infection recently? Mycoplasma, hepatitis C, syphilis, parvovirus, pneumococcal sepsis, meningococcemia, and tuberculosis bacillus have all been associated with LR
Have any new drugs been initiated? Amantadine, norepinephrine, interferon, minocycline and quinine represent the most usually reported causes of drug-induced livedo reticularis. Rarely, gemcitabine, heparin, and bismuth have also been reported to induce livedo reticularis.
Has the patient recently undergone any invasive vascular diagnostic procedure? Arteriography catheterization may provoke cholesterol emboli syndrome and thus induce livedo reticularis.
A detailed systemic review can also be helpful in uncovering any systemic conditions such as autoimmune disease.
Physical examination for livedo reticularis diagnosis
Characteristically livedo reticularis findings during physical examination may be:
- Netlike, mottled pattern of blue-red to violaceous interconnecting skin patches of rings
- In the case of physiologic livedo reticularis, discoloration is ususally fainter and networks are narrow-based. It also has predominant lower extremities localization. It is transient and resolves after warming.
- In the case of primary or idiopathic livedo reticulatis discoloration is usually faint and network is narrow-based. Condition persists, and re-warming usually doesn’t help.
- In the case of Secondary livedo reticularis which is caused by underlying condition, discoloration is intensive and network is wide or in starburst patterns. Buttocks and upper extremities may be also affected.
- Presence of necrosis, purpura, and ulceration should be evaluated if there is any suspicion to vasculitis or calciphylaxis or obstructions of the small vessels with either emboli or thrombosis.
- Palpable purpura is the most common finding of physical examination in the cases small-vessel vasculitis.
- Nodules and ulcerations on lower extremities are findings that may indicate condition called polyarteritis nodosa.
- If there are signs of purpuric eruptions in various stages from papules to hyperpigmented macules, this may suggest cryoglobulinemia.
- Malar erythema, photodistributed eruption, photosensitivity, discoid lesions, alopecia, and arthralgia may be the signs of SLE.
- Keratoconjunctivitis, scaly annular erythema and xerostomia on face and neck are commonly associated with Sjögren’s syndrome
- Skin fibrosis and sclerosis, matted telangiectasias, Raynaud’s phenomenon, dilated capillary loops in nail fold and calcinosis cutis may be the signs of Systemic sclerosis.
Biopsy for livedo reticlularis
Since sampling errors are common during biopsy procedure, sensitivity is very poor. In order to capture medium-sized vessels incisional biopsy to fascia should be considered. Ideally, the sample passes through the blanched as well as bluish parts.
Microscopic pathology is usually not observable in the case of physiological or primary livedo reticularis. Secondary caused livedo reticularis may vary depending on etiology and may include:
- Vasculitis in the case of fibrin accumulation in vessel walls and leukocytoclasia
- Hypercoagulable states in the case of Intraluminal thrombosis
- Calciphylaxis in the case of calcium deposition in vessel walls
- Monoclonal cryoglobulinemia in the case intravascular eosinophilic plugging
- Cholesterol emboli syndrome in the case of cholesterol clefting
Serology tests for livedo reticularis
Serologic workup depends on etiology and should be directed by the clinical assessment
Primary and physiologic livedo reticularis: No laboratory workup suggested
Secondary livedo reticularis may include following tests for next conditions:
Systemic lupus erythematous:
- Elevated titer ANA (most sensitive)
- Anti-Smith antibodies (specific)
- Aanti-dsDNA (specific) antibodies
- CBC for anemia and thrombocytopenia
- Complement levels (C3, C4) for hypocomplementemia.
Sjögren’s Syndrome:
- Anti-Ro (SS-A), usually positive in 50% of patients with primary Sjögren’s Syndrome
- Anti-La (SS-B), usually positive in 50% of patients and may be lacking if Anti-Ro antibody is also absent.
- ANA may also be positive.
- Elevated titer rheumatoid factor
- CBC for anemia.
Systemic Sclerosis:
- Anti-centromere and anti-topoisomerase I DNA (Scl70) antibodies may be positive in scleroderma.
Vasculitis:
- CBC serology and anti-streptococcal antibodies.
- ESR serology
- ANCA serology
- hepatitis B and C testing
- Urinalysis and basic metabolic panel in cases of suspected PAN for expected proteinuria and acute renal failure.
Cryoglobulinemia:
- Serum cryoglobulins, UA, CBC, SPEP, serum chemistries, liver function tests.
- Plasma cryofibrinogens are fibrinlike proteins
- Blood must be collected in an anticoagulated tube and centrifuged at 37 C.
Infections:
Infections may be related with the presence of livedo reticularis. Consider checking cold agglutinins in suspected cases of mycoplasma infection. Check viral hepatitis panels in suspected cases of PAN.
Hypercoagulable states:
- Anti-cardiolipin antibodies ( IgA, M, G)
- Beta-2 glycoprotein antibodies (IgA, M, G)
- Lupus anticoagulant
- Antithrombin III
- Protein C
- Protein S
- Factor V Leiden mutation
- Homocysteine levels
What is Cutis marmorata telangiectatica congenita?
This rare condition is present at birth and is usually localized only to one lower extremity, though it may be also found on the abdomen or be generalized. Telangiectasias as well as other vascular anomalies may be also present. Limb or body asymmetry may also be present. Cutaneous findings usually aim to recover spontaneously over first few years of life.
Livedo reticularis treatment
If there are no systemic manifestations of livedo reticularis then therapy except avoiding cold is not needed as physiological or primary LR usually is resolved spontaneously. Use of compression stockings and limb elevation may be helpful.
Vasodilatators drugs may also be considered for a short-term use. Nifedipine, calcium-channel blocker has been shown to be effective for Raynaud’s phenomenon, so it may be also effective for vasospastic livedo reticularis.
Pentoxifylline is a vasodilatator that increases deformability of erythrocytes and may also reduce platelet aggregation and activation thus it may be beneficial in primary or secondary LR. As a last option, short-term vasodilator therapy may be given off-label in the patient for cosmetic purpose.
Therapy for Livedo racemosa should be directed according to underlying disorder. Patients with livedo racemosa and the antiphospholipid antibody syndrome with thrombosis need anticoagulation therapy.
Treatment of livedoid vasculopathy is not promising but potentially beneficial drugs may include different anticoagulants and antiplatelet agents, pentoxifylline, immunosuppressants, danazol, and tissue plasminogen activator. The combination of hyperbaric oxygen, psoralen and ultraviolet A light therapy have also been shown to be successful in cases with livedoid vasculopathy.
Inflammatory manifestations of LR should be treated with anti-inflammatory agents, including NSAID-s, systemic corticosteroids and systemic immunosuppressants.
Treatment of hyperviscosity conditions such as Polycythemia vera should be focused on maintaining a low hematocrit. Thus, phlebotomy alone is effective though patients may also receive hydroxyurea or anagrelide. A low-dose aspirin may be also helpful.
If cholesterol embolism is doubted as the underlying cause of livedo reticularis, long-term management is directed toward removing and modifying precipitating risk factors. Anticoagulants use in that case is controversial, since it may disrupt fibrin caps that hold cholesterol plaques in place attached to the vessel wall.
The treatment of Sneddon syndrome is also controversial. Corticosteroids have been proved to be ineffective. Generally, anticoagulation therapy is recommended. Management should be coordinated with rheumatology and neurology.