Contents
- What is TEVA 834 pill?
- Active ingredient of TEVA 834 pill and identification
- TEVA 834 pill chemistry
- TEVA 834 pill uses
- TEVA 834 pill legal status
- TEVA 834 pill mechanism of action
- TEVA 834 pill pharmacokinetics
- TEVA 834 pill use during pregnancy and breastfeeding
- TEVA 834 pill side effects
- How long TEVA 834 pill stays in the system?
- TEVA 834 pill contraindications
What is TEVA 834 pill?
TEVA 834 is an imprint on a pill identified as white in a round shape. The pill contains clonazepam as an active ingredient in the dose of 2 mg. Clonazepam is an anticonvulsant benzodiazepine drug. It is supplied by Teva Pharmaceuticals USA.
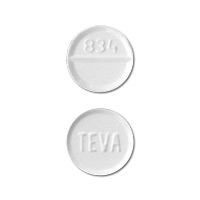
- Imprint: TEVA 834
- Strength: 2 mg
- Color: White
- Shape: Round
- Availability: Prescription
- Drug Class: Benzodiazepine anticonvulsants, Benzodiazepines
- Pregnancy Category: D – Positive evidence of risk
- CSA Schedule: Schedule 4 – Some potential for abuse
- Labeler / Supplier: Teva Pharmaceuticals USA
- Inactive Ingredients: corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone
Active ingredient of TEVA 834 pill and identification
An active ingredient of TEVA 834 pill is clonazepam. Clonazepam is a benzodiazepine drug. It affects brain chemicals that may be unbalanced. Clonazepam is also an anti-seizure or anti-epileptic drug, usually prescribed for absence seizures or Lennox-Gastaut syndrome in adults and children. It also used to treat panic disorder (including agoraphobia) in adults.
It appears as an off-white to a light yellow crystalline powder of a faint odor with a solubility in water of <100 mg/L at 25 deg.
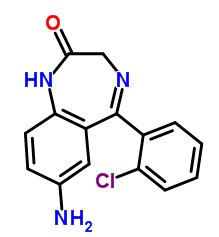
TEVA 834 pill chemistry
Clonazepam:
IUPAC name: 5-(2-chlorophenyl)-7-nitro-2,3-dihydro-1H-1,4-benzodiazepin-2-one
Molecular formula: C15H10ClN3O3
Molecular weight: 315.711 g/mol
Molecular structure:
Drug class: Clonazepam belongs to the class of organic molecules known as 1,4-benzodiazepines which contains a benzene ring fused to a 1,4-azepine.
TEVA 834 pill uses
FDA approved indications for TEVA 834 pills are:
- Akinetic seizures
- Burning Mouth Syndrome
- Gilles de la Tourette’s Syndrome
- Lennox-Gastaut Syndrome (LGS)
- Mixed manic depressive episode
- Panic Disorders
- Rapid Eye Movement Sleep Disorder
- Restless Legs Syndrome (RLS)
- Tardive Dyskinesia
- Tremor, Essential
- Acute Manic episode
- Myoclonic seizures
- Refractory absence seizures
TEVA 834 pill legal status
Under the Controlled Substance Act (CSA) clonazepam is a Schedule 4 drug which means that the medicine has a low potential for abuse relative to the drugs in Schedule 3 and abuse of the drug may lead to limited physical dependence or psychological dependence.
TEVA 834 pill mechanism of action
Clonazepam produces allosteric interactions between CNS benzodiazepine receptors and GABA receptors and potentiate the effects of GABA. As GABA is an inhibitory neurotransmitter in the brain, the result is increased inhibition of the ascending reticular activating system. In this way, clonazepam blocks the cortical and limbic arousal following stimulation of the reticular pathways.
TEVA 834 pill pharmacokinetics
Absorption and Distribution. TEVA 834 pill is rapidly and completely absorbed after oral administration. The absolute bioavailability is about 90%. Maximum plasma concentrations of TEVA 834 pills are reached within 1 to 4 hours after oral administration. The drug is approximately 85% bound to plasma protein.
Metabolism and excretion. TEVA 834 pills have extensive metabolism, with less than 2% of unchanged clonazepam being excreted in the urine. Biotransformation occurs predominantly within the reduction of the 7-nitro group to the 4-amino derivative which is then acetylated, hydroxylated and glucuronidated. CYP-450 including CYP3A, may play an important role in metabolic reactions of clonazepam reduction and oxidation.
The elimination half-life time of this drug is typically 30 to 40 hours. Pharmacokinetics are dose-independent within the dosing range. There is no evidence that TEVA 834 pill induces its own metabolism or that of other drugs in humans.
TEVA 834 pill use during pregnancy and breastfeeding
TEVA 834 pills should not be used during pregnancy unless the benefit outweighs the risk. It is classified in a D category by the US FDA pregnancy list of drugs meaning that child born to a mother taking this drug may be at risk for withdrawal symptoms. A mother should be warned of the potential risks to the fetus and counseled to discontinue the drug prior to becoming pregnant.
Studies showed the risks of neonatal flaccidity, hypothermia and respiratory and feeding difficulties in children born to mothers who have been taking this drug late in pregnancy.
Clonazepam may be excreted into milk and occasionally may provoke sedation in breastfed infants, especially when given with other CNS depressants. An infant should be monitored for drowsiness, adequate weight gain, and developmental milestones.
TEVA 834 pill side effects
Clonazepam may cause following side effects:
Common side effects:
- Dizziness
- Confusion
- Increased saliva
- Muscle aches
- Frequent urination
- Blurred vision
- Loss of interest in sex
- Fatigue
- Depression
- Memory loss
- Nervousness
- Upper respiratory congestion or infection
- Constipation
- Decreased appetite
Serious side effects:
- Severe rash or hives
- Trouble breathing or swallowing
- Swelling of your face, lips, or tongue
- Chest pain
- Worsening depression
- Thoughts of suicide
How long TEVA 834 pill stays in the system?
To determine how long TEVA 834 pill will remain in the body after the last dose, it is necessary to consider the elimination half-life of clonazepam. Clonazepam has a long elimination half-life time in the range of 30 to 40 hours, meaning that it will take about 1 and 2 days just to eliminate 50% of the drug from the body. According to some estimations 6.88 to 9.17 days are needed after the final dose.
Other data suggest that clonazepam may also have a wider-ranging half-life of 18.7 hours to 60 hours. If this is the case, it could take between 4.29 and 13.75 days for complete TEVA 834 pill excretion.
In addition, as clonazepam is metabolized within the liver, it forms a metabolite known as 7-aminoclonazepam. According to some studies, the half-life of 7-aminoclonazepam is either shorter than or similar to that of clonazepam. Therefore, the body should clear clonazepam as well as its 7-aminoclonazepam metabolites within 2 weeks of cessation.
TEVA 834 pill contraindications
TEVA 834 pill is contraindicated in patients with the following conditions:
- History of sensitivity to benzodiazepines
- Clinical or biochemical evidence of significant liver disease
- Acute narrow angle glaucoma (it may be used in patients with open angle glaucoma who are receiving appropriate therapy).
Following precautions should be made:
- When used in patients in whom several different types of seizure disorders coexist, clonazepam may increase the incidence or precipitate the onset of generalized tonic-clonic seizures (grand mal).
- Periodic blood counts and liver function tests are advisable during long-term therapy with clonazepam.
- Patients with renal, liver and respiratory diseases should talk with their doctor before isng this drug.
“4839 V pill – Drug class, street names, uses, strength, side effects, overdose, price“