Contents
- What is Trazodone? What is Trazodone used for?
- Trazodone structure
- How Trazodone work in the body?
- Trazodone possible side effects
- Trazodone dosage
- Trazodone withdrawal
- Trazodone addiction and abuse
- Pharmacokinetics of Trazodone
- Trazodone drug interactions
- Trazodone during Pregnancy & Breastfeeding
- Trazodone overdose
What is Trazodone? What is Trazodone used for?
Trazodone is antidepressant medicine that is used to treat depression. Trazodone is classified in the group of antidepressant drugs called serotonin uptake inhibitors. These antidepressants work by increasing serotonin levels in the brain. It is indicated for patients with major depressive disorders and other types of depressive disorders.
In general, trazodone is more useful in depressive disorders that are combined with insomnia and anxiety. This medicine may be also prescribed to treat anxiety, schizophrenia, or uncontrolled movements that may occur as a side effect of other drugs.
A study that was conducted in 2014 and published in the American Journal of Geriatric Psychiatry found that trazodone was effective in treating sleep disorders in patients with Alzheimer’s disease. Trazodone works by improving nighttime sleep without affecting brain function and thinking. Also, studies found that drug did not cause daytime sleepiness or naps.
In some cases trazodone can be prescribed for the treatment of pain, fibromyalgia and bipolar disorder. Trazodone is available in the form of immediate and extended release tablets in doses of: 50 mg, 100 mg, 150 mg and 300 mg. FDA approved trazodone in 1981 under the brand name Desyrel. It is available in generic form as Trazodone and in the only brand name remaining for trazodone called Oleptro.
Trazodone structure
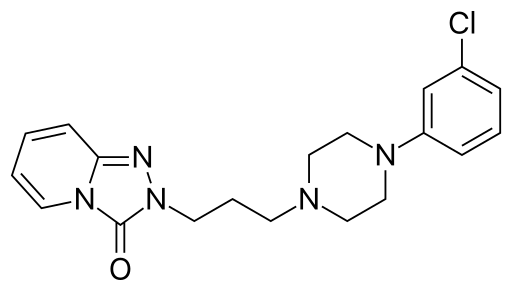
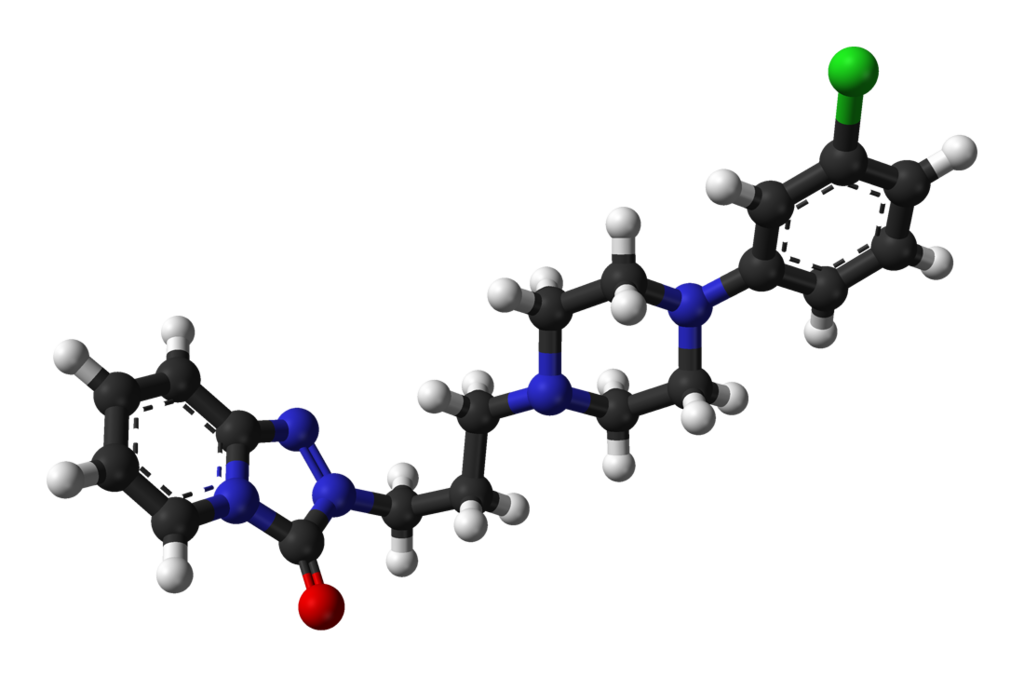
- Trazodne IUPAC name is 2-(3-[4-(3-Chlorophenyl)-1-piperazinyl]propyl)[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one.
- Its molecular formula is C19H22ClN5O with a molar mass of 371.8641 g/mol
- This compound belongs to the class of organic compounds that are known as phenylpiperazines. It contains a phenylpiperazine structure that has piperazine bound to a phenyl group.
How Trazodone work in the body?
Trazodone works by binding to brains 5-HT2 receptor. At high doses trazodone is serotonin agonist and in low doses this drug works as antagonist of same receptors. Trazodone’s antidepressant activity most likely is a result of serotonin reuptake inhibition, becasue of the serotonin reuptake pump blockade located on the presynaptic neuronal membrane.
If it is used for long term, it may also affect postsynaptic neuronal receptors. Trazodone sedative actions can be also the result of alpha-adrenergic antagonism and modest histamine blockade at H1 receptor. It is also weak antagonist of presynaptic alpha2-adrenergic receptors and strong antagonist of postsynaptic alpha1 receptors. Trazodone has no effects on the reuptake of norepinephrine or dopamine within the CNS.
Trazodone possible side effects
Trazodone may cause following side effects with following incidence:
- Blurred vision (5-15%)
- Dizziness (20-28%)
- Drowsiness (24-40%)
- Dry mouth (15-34%)
- Fatigue (6-15%)
- Headache (10-33%)
- Nausea/vomiting (10-21%)
- Constipation (7-8%)
- Edema (3-7%)
- Confusion (5-6%)
- Disorientation (<2%)
- Incoordination (2-5%)
- Nasal congestion (3-6%)
- Orthostatic hypotension (<7%)
- Syncope (<5%)
- Tremor (1-5%)
- Weight change (5%)
- Ejaculation disorder (2%)
- Decreased libido (1-2%)
Incidence <1%
- Priapism
- Sedation
- Alopecia
- Anxiety
- Acne
- Anemia
Trazodone warnings and precautions
- The FDA notes a black-box warning on trazodone, because taking this drug may increase the chance of becoming suicidal. The risk of suicide is greatest at the beginning of treatment or when the dose of trazodone is increasing or decreasing. Suicide risk may be higher for patients younger than 24. At the beginning of the therapy with trazodone, depression may become worse before it gets better.
- Doctor should monitor you carefully for symptoms such as: irritability, panic attacks, extreme worry, aggression, acting without thinking, restlessness, abnormal excitement, or any thoughts of suicide when you start the drug.
- Patients should also let their friends and family members to know if they have these symptoms or if their depression is worsening. If patient have any suicide thoughts or if a friend or family member thinks they are acting strangely, call a doctor right away.
- Trazodone can cause withdrawal symptoms if it is stopped suddenly, these symptoms may include anxiety, agitation, and difficulty sleeping.
- Children or teens should never take trazodone.
- Patients should tell their doctor if they have or have ever had:
- Heart problems
- History of substance abuse
- Low or high blood pressure
- Any mental health disorders
- Anemia
- Diarrhea or vomiting
- Liver problems
- Kidney problems
- Cancer
- Any patient taking this drug should let their doctor know about any family history of suicide.
- If you are a man, let your doctor know if you have any problem with an erection that will not go away.
- It’s important to tell your doctor if you have symptoms of irritability, aggression, extreme worry, panic attacks, restlessness, acting without thinking, abnormal excitement, or any thoughts of suicide while on trazodone.
Trazodone dosage
Trazodone should be started at a low-dose and this dose should be increased gradually. If drowsiness occurs, this may require the usage of a major portion of the daily dose at bedtime or a dose reduction. Trazodone should be taken a short time after a meal.
Initial treatment: It has been suggested that initial trazodone dose should be 150 mg/day in divided doses. The dose may be increased by 50 mg/day every 3-4 days. The maximum dose should not exceed 400 mg per day in divided doses. The highest allowable dose for serious cases should not exceed 600 mg per day in divided doses.
Maintenance Treatment: When appropriate response after trazodone use has been achieved, doses may be reduced gradually with adjustment depending on therapeutic response. When doses are reduced, patients should be carefully monitored for withdrawal symptoms when discontinuing treatment with Trazodone. The dose should be gradually reduced whenever possible.
Trazodone withdrawal
Like any other central nervous system (CNS) depressant, sudden abrupt of trazodone can cause withdrawal symptoms. Although labels of manufacturers do not warn about withdrawal symptoms, they may occur, and most commonly in patients taking higher doses of trazodone during longer period of time.
Also patient’s non-compliance with this drug treatment regimen, with missing doses of trazodone for several days may precipitate withdrawal symptoms. Patients who experience withdrawal usually find these symptoms as a reminder that they have missed some doses, while for others this unpleasant experience could cause them to permanently stop taking this drug.
Trazodone withdrawal symptoms usually include:
- Agitation
- Anxiety
- Confusion
- Dizziness
- Headache
- Insomnia
- Irritability
Usually, these symptoms do not require medical attention. However, in some cases it is possible for some patients to develop severe discontinuation symptoms causing syndrome state that can last for several weeks and could cause significant adverse effects.
Severe emotional or psychological disturbance are considered as more serious symptoms of trazodone withdrawal. Patients exhibiting such problems may need urgent medical attention to manage this withdrawal and to prevent possible harm. In rare cases, patients may also develop withdrawal symptoms such as mood swings, aggression and irritability. These symptoms may compromise the patient’s safety.
Patients who are taking trazodone as a sleep aid, abrupt discontinuation can lead to a insomnia recurrence or even worsen primary condition.
Trazodone and weight changes
The true trazodone’s effect on the patient’s body weight is quite complex. In some patents, this drug causes weight loss while in others weight gain. Some studies have been found that weight loss is more common side effect of trazodone, with incidence of 5.7 % in patients who have taken this drug.
However, it has been also found that weight loss last for a short-term period after eventually may lead to weight gain if trazodone is used during a long-term period.
It is ebntirely clear the mechanism how trazodone causes weight loss. Many patients find eating as a defense mechanism to cope up with the depression. Once depression is relieved, patients may start again with normal eating pattern and weight loss becomes evident.
Weight gain is known as a common depression symptom hence weight loss can be a welcome one. Trazodone can cause gastrointestinal side effects in some patients, such as diarrhea, nausea and loss of appetite. These side effects may be the cause of possible weight loss.
One of trazodone side effects may be also weight gain, and it is most commonly caused after a long-term period of treatment. Medical experts believe that trazodone can cause weight gain as a result of a variety of biochemical mechanisms and drugs pharmacologic. Like other antidepressant drugs, this drug can also increase the appetite of a person.
It is known that loss of appetite can be also a symptom of depression in some patients, so once symptoms of depression are relieved the person’s appetite is restored. In patients whose symptoms of depression are accompanied by appetite loss increase in weight may be expected in gradual manner. In some cases, trazodone can lead to craving for certain types of foods such as fat or carbohydrates.
Also many health professional believe that the increase in serotonin levels can lead to increased appetite. Clinical studies showed that the incidence of weight gain has been reported in about 4.5% of patients taking trazodone, thus it is considered as a common side effect.
Trazodone addiction and abuse
Although Trazodone has not been adequately studied in preclinical or clinical studies for its potential effects of abuse, there are no indications of drug-abusive behavior that have been studied so far. However, it is always difficult to find out the extent to which a CNS-active drug will be misused, diverted, and abused. Health profession should especially monitor patients who had history of drug abuse, while they are on trazodone therapy.
Pharmacokinetics of Trazodone
Absorption. Trazodone is well absorbed after oral use without selective cumulation in any tissue. If Trazodone is taken shortly after food consumption, increased amounts of the drug will be absorbed with reduced maximum concentration and a lengthening in the time that ti needed for achieving maximum concentration.
Peak plasma levels usually occur after one hour of taken trazodone dose when the drug is taken on empty stomach is or after 2 hours if trazodone is taken with food.
Metabolism and Elimination. Trazodone is metabolized, through oxidative degradation, to an active metabolite, m-chlorophenylpiperazine by CYP3A4 liver enzyme. Other metabolic pathways that may be also involved in the trazodone’s metabolism have not been well established yet. Trazodone has extensive metabolism and less than 1% of an taken oral dose is excreted unchanged in the urine.
Protein Binding. Trazodone protein binding rate is 89 to 95%
Trazodone drug interactions
1. Trazodone + MAOIs drugs
Patients who are receiving monoamine oxidase inhibitor (MAOI) drugs in combination with serotonergic drugs such as trazodone, there are a data of serious and in some cases fatal side effects hyperthermia, myoclonus, rigidity, autonomic instability and changes in mental status changes that may include extreme agitation that could progress to delirium and coma.
These adverse reactions have also been reported in patients who have discontinued antidepressants and have been started on an MAOI. In some cases there are reports of neuroleptic malignant syndrome. Limited animal studies data also showed that combination of serotonergic antidepressants and MAOIs work synergistically and may cause high blood pressure and behavioral excitation.
Therefore, trazodone should not be used in combination with an MAOI drugs such as phenelzine, selegiline, tranylcypromine, etc or within 14 days of discontinuing therapy with an MAOI drugs. Similarly, at least 14 days are needed after stopping trazodone tablets before starting an MAOI.
- Trazodone + CNS depressants
Trazodone may enhance the effects of alcohol, barbiturates, and other CNS depressants.
- Trazodone + Cytochrome P450 3A4 Inhibitors
Studies of in vitro drug metabolism have been found that there is a potential for drug interactions when Trazodone is given in combinations with cytochrome CYP3A4 inhibitors drugs such as ritonavir, Clarithromycin , erythromycin, itraconazole, ketoconazole, verapamil, diltiazem, grapefruit juice, .
Adverse effects that are described as a result of this interaction may include hypotension, nausea and syncope. It is likely that CYP3A4 inhibitors will lead to increasege in Trazodone plasma concentrations with the high potential for adverse effects. If Trazodone is used in combination with a potent CYP3A4 inhibitor, cardiac arrhythmia’s risk may be increased thus lower doses of Trazodone should be considered.
- Trazodone + Cytochrome P450 3A4 Inducers
Carbamazepine, Hypericum perforatum (St. John’s wort), phenytoin, phenobarbital, rifampin are drugs known as potent inducers of CYP3A4 liver enzyme. Following coadministration of such drugs with trazodone may reduce plasma concentrations of Trazodone and its active metabolite, thus lowering the effects of trazodone taken dose. Patients should be closely monitored in order to be found if there is a need for an Trazodone’s increased dose when both drugs are taken.
- Trazodone + Digoxin or Phenytoin
Increased plasma levels of digoxin or phenytoin and thus side effects have been reported in patients receiving Trazodone in combination with one of these drugs. Serum levels monitoring and dose adjustment may be required when these drugs are used together.
- Trazodone + NSAIDs, Aspirin, Warfarin and other anticoagulants
Due to a possible link between serotoninergic drugs and gastrointestinal bleeding, patients should be carefully monitored for the potential risk of bleeding associated with the concomitant use of Trazodone and NSAIDs, aspirin, or other drugs that may affect coagulation or bleeding. There are reports of altered prothrombin times if warfarin and trazodone are used together.
Trazodone and alcohol
Patients who are on trazodone therapy have to avoid alcohol. Manufacturers warn patients of the potential interaction between trayodone and alcohol. Both of these substances work by affecting the same brain chemicals and neurotransmitters, thus increasing potential risk of side effects.
Alcohol may increase the risk of drowsiness and dizziness as common trayodone’s side effects while trazodone may enhance the effects of alcohol. Many factors influence the severity of these interaction side effects, however, trazodone dosage and alcohol amount that are taken are the most imortant one.
Combining alcohol and trazodone can lead to several side effects such as
- Dizziness
- Drowsiness
- Impaired judgment and thinking
- Decreased psychomotor skills
- Delayed reaction time
However, depending on health state and dosage, doctor may allow light to moderate amount of alcohol, but only if patient have learned the specific effects of trazodone. Patients who choose to drink alcohol while they are on trazodone therapy should limit their alcohol intake to no more than one to two drinks. Safely alcohol intake can be for example 5 ounces of wine or 12 ounces of beer.
Trazodone during Pregnancy & Breastfeeding
Studies showed that about 13 % of pregnant women experience depression symptoms at some point during pregnancy. Use of trazodone and other antidepressants during pregnancy and breastfeeding is in question and concern because of the potential risks that these drgs can cause to the unborn child.
Opposite from that, many health professionals believe that mother’s emotional status is as equally important as the physical health because depression can have greatly affect at the pregnancy outcome.
Trazodone use during pregnancy
FDA classifies trazodone in a Pregnancy Category C list of drugs used during pregnancy which means that trazodone has not been fully studied yet in pregnant patients however animal studies suggest that this drug can cause harm on the unborn fetus. Under doctor supervision, trazodone may still be given to pregnant if there are more benefits that outweigh risks.
Animal studies on rats showed that trazodone increases the risk of miscarriages. Other study on pregnant rabbits, have been shown that trazodone increases the risk of birth defects or congenital anomalies. But, there are still no adequate data or well-controlled studies that can evaluate trazodone safety use in pregnant women.
If patients become pregnant during trazodone used, he/she should contact healthcare provider right away. It is also advisable to discuss with healthcare provider any plans of becoming pregnant during treatment with trazodone. Since depression during pregnancy may increase health risks on the unborn child, healthcare provider may consider if benefits of trazodone use outweigh risks before making any recommendation.
Can Breastfeeding Mothers Take Trazodone?
Dtudies on animal have been shown that small amounts of trazodone can pass into the breast milk. It has been suggested that trazodone may also pass into human milk. But this trazodone amounts in milk are very low thus it is not expected that any serious side effects may be caused on infant, especially in those who are older than 2 months or when trazodone is used in doses lower than 100 mg.
One case report showed that infant breastfeed by a mother who were on trazodone therapy 200 mg per day during 12 weeks, from fourth week of age, found no adverse effects on the child growth and development. Other studies show the same results.
However, whenever trazodone is used during breastfeeding, caution should be taken in order to avoid side effects on the infant.
Can Trazodone cause arrhytmias?
There are some reported cases of cardiac arrhythmias related with trazodone treatment, in patients with pre-existing valve issues and in patients with negative family or personal histories of cardiac disease.
Trazodone can prolong the QT interval of cardiac cells and cause problems with heart rhytm. It is known that there are also other drugs that can cause certain type of arrhythmia known as torsade de pointes which is related with unexplained and sudden death.
The relationship between prolongations of QT interval is clearest for when trazodone is used in high doses but it is also possible that smaller QT prolongations may also be increased with normal doses in predisposed patients with hypomagnesaemia, or in those with genetic predisposition of prolonged QT.
What is priapism? Can trazodone cause priapism?
Priapism is a medical condition which is characterized with painful erections with duration of 6 hours and more. Cases of priapism are reported in men receiving trazodone. If priapism is not treated immediately, it can result in irreversible damage of the erectile tissue. This relatively rare, but dramatic, side effect is most likely associated with the antagonism of α-adrenergic receptors. There are more of 200 reported cases.
It has been estimated that the incidence of priapism is about 1 : 6,000 in male population treated with trazodone. The risk for this side effect is the greatest during the first month treatment with doses lower than 150 mg/ trazodone. Studies have also described trazodone-associated psychosexual side effects in women, priapism of the clitoris, including increased libido and spontaneous orgasms.
Men who experience an erection lasting longer than 6 hours during trazodone treatment, no matter if it is painful or not, should immediately discontinue the drug and seek immediate emergency attention.
Trazodone should be used with caution in patients who have conditions that might predispose priapism (for example sickle cell anemia, multiple myeloma or leukemia), or in men with anatomical deformation of the penis (such as cavernosal fibrosis, angulation or Peyronie’s disease).
Can trazodone cause bleeding?
Postmarketing reports have been shown a link between use of meidicnes that may interact with serotonin reuptake inhibitors and the occurrence of gastrointestinal bleeding. While there are no association between Trazodone and bleeding events, patients should be still cautioned about possible risk of bleeding linked with together use of Trazodone and NSAIDs drugs, aspirin, or other meidines that may affect coagulation or bleeding.
Other bleeding events that have been found that are related to SSRIs and SNRIs, but not particularly with trazodone have been ranged from ecchymosis, epistaxis, hematoma and petechiae to life-threatening hemorrhages.
Can trazodone cause low sodium levels in the body?
Hyponatremia, or low sodium-levels in the body may occur as a result of antidepressants treatment. Most commonly, hyponatremia appears to be the cause of health condition known as the syndrome of inappropriate antidiuretic hormone secretion (SIADH). There have been reported cases of serum sodium lower than 110 mmol/L after trazodone use.
Elderly patients and also patients taking diuretics or who are otherwise volume-depleted can be at greater risk. Discontinuation of Trazodone hydrochloride tablets should be considered in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include difficulty concentrating, headache, confusion, memory impairment, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe cases have included hallucination, seizure, coma, syncope, respiratory arrest and death.
Trazodone overdose
There have been reported cases of high doses of trazodone causing serotonin syndrome. There are also reports of serotonin syndrome in patients taking multiple doses of SSRIs with trazodone.
However, studies also showed that Trazodone is relatively safer than TCAs, MAOIs, and some second-generation antidepressants from the point of overdose. Fatal cases are reported very rare, and uneventful recoveries were reported after ingestion of trazodone’s in doses higher 6,000–9,200 mg. One report showed that 9 of 294 cases of overdose were fatal, and all nine patients had also taken different central nervous system depressants.
When trazodone overdoses occur, physicians should carefully monitor for effect of serious hypotension. Report of a trazodone overdose that was fatal showed that torsades de pointes with complete atrioventricular block was developed along with subsequent multiple organ failure. Trazodone plasma concentration was 25.4 mg/L.
There is no specific antidote substance for trazodone. Overdosage management should be symptomatic and supportive. Any patient suspected of having taken an overdosage should be evaluated at a hospital as soon as possible. Use of Activated charcoal and forced diuresis may be useful in facilitating elimination of the drug. Gastric lavage has been also shown to be useful unless done during the first hour after intake.
Trazodone and bipolar disorder screening
A depressive episode may be just the initial manifestation and symptome of bipolar disorder. It is believed since it is not yet confirmed in clinical studies that treating depressive episode of bipolar disoreder with an antidepressant such as trazodone as individual therapy may increase the probability of precipitation of a mixed-manic episode in patients who are at high risk for bipolar disorder.
It is unkown, whether any of these symptoms will manifest clinical worsening and suicide risk. However, prior to begining of the treatment with trazodone, patients with depressive symptoms should be screened to determine if bipolar disorder exist.
Screening should include patients psychiatric history in detail, including a history of eventual suicide in family, bipolar disorder or depression. All of this should be done, because trazodone is not approved for use in bipolar disorder treatment.
Can Trazodone cause cognitive and motor impairment?
Trazodone may cause sedation and somnolence thus it may have negative effects on the mental and/or physical ability that is needed for the potentially hazardous tasks. Patients should be cautioned about operating hazardous machinery, including vehicles, until they are certain that the drug treatment does not affect them negatively.
Can patients with glaucoma use trazodone?
The dilation of pupills that occurs after use of antidepressant drugs including Trazodone may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Thus, this medicine should not be used in patients with glaucoma
Can Trazodone cause Serotonine Syndrome? Is Serotonine Syndorme life threatening condition?
Antidepressants can cause potentially life-threatening condition called serotonin syndrome or neuroleptic malignant syndrome. This serious condition have been reported with antidepressants alone but most commonly with combination of different antidepressants.
It may also occur after Trazodone therapy, but particularly with trazodone’s concomitant use with different serotoninergic drugs such as SSRIs (Citalopram, Fluvoxamine, Escitalopram, Paroxetine, Sertraline, Fluoxetine), SNRIs (venlafaxine, duloxetine, atomoxetine) and triptans (sumatriptan, zolmitriptan, fruvatriptan, noratriptan) and with drugs or substances that may impair serotonin’s metabolism such as monoamine oxidase inhibitors drugs or with antipsychotic drugs or other dopamine antagonists.
Serotonin syndrome symptoms most usually include mental status changes such as hallucinations, agitation and coma, autonomic system issues such as tachycardia, hyperthermia, labile blood pressure, neuromuscular problems including hyperreflexia, motor incoordination and gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
In its most severe form, this syndrome can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Treatment with Trazodone and any concomitant serotonergic or antidopaminergic drugs, including antipsychotics, should be immediately discontinued if the these reactions occur and supportive symptomatic treatment should be initiated.
“Can you overdose on zolpidem?
“Is Trazodone similar to Xanax?“