Contents
- What is travoprost?
- Travoprost IUPAC name, molecular formula, weight, structure and drug class
- What is the mechanism of action of travoprost?
- What is the pharmacokinetics of travoprost?
- How long travoprost stays in your system?
- Travoprost dosage
- What are the side effects of the travoprost?
- How to use travoprost ophthalmic?
- How to apply travoprost eye drops?
- Can travopost be used for eyelashes growth?
- Can I use travoprost in pregnancy?
- Travoprost carcinogenesis, mutagenesis, impairment of fertility
- Can I use travoprost during breastfeeding?
- Can I use travopost for hair growth?
- Can travoprost be used for management of open-angle glaucoma and ocular hypertension?
- Can travoprost be taken with tafluprost?
- Travoprost effects on pigmentation
- Can travoprost be given in patient with macular edema?
- Can travoprost provoke bacterial keratitis?
- Travoprost use with contact lenses
- Can travoprost be given in patients with uveitis?
- Can travoprost be given to a patient with liver or renal disease?
What is travoprost?
Travoprost is an ophthalmic solution which is used for treating the conditions like increased intraocular pressure and is also used for controlling the progression of glaucoma or ocular hypertension. Travoprost is a synthetic analog of prostaglandin F2alpha.
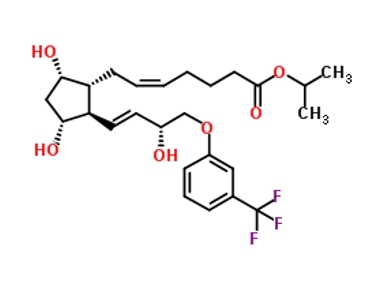
Travoprost IUPAC name, molecular formula, weight, structure and drug class
Molecular formula: C26H35F3O6
Molecular weight of travopost: 500.555 g/mol
IUPAC Name: propan-2-yl (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-enyl]cyclopentyl]hept-5-enoate
Molecular structure:
Drug class: This compound belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid.
What is the mechanism of action of travoprost?
Travoprost is known to reduce the intraocular pressure by enhancing the drainage of aqueous humor as it is a selective FP prostanoid receptor agonist. The drainage of the aqueous humor is done by increasing the uveoscleral outflow and to a lesser extent, trabecular outflow facility.
What is the pharmacokinetics of travoprost?
Travoprost is systemically absorbed when administered to the eye and is metabolized by esterases as it is an isopropyl ester prodrug. It is hydrolyzed or metabolized in the cornea and transferred to its free acid which is biologically active.
The topical ocular dose of the travoprost (less than 2%) is known to get excreted via urine as travoprost free acid with 4 hours of the administration. The elimination half-life of the travoprost free acid is 45 minutes.
How long travoprost stays in your system?
The elimination half-life of travoprost is 45 minutes (after it gets converted into free acid) and the elimination of the drug is equals to five-time to that of the elimination half-life, therefore, it will be out of your system in 4 to 5 hours completely.
Travoprost dosage
The recommended dosage is one drop in the affected eye(s) once daily in the evening. Travoprost ophthalmic solution should not be administered more than once daily since it has been shown that more frequent administration of prostaglandin analogs may decrease the intraocular pressure lowering effect.
Reduction of the intraocular pressure starts approximately 2 hours after the first administration with maximum effect reached after 12 hours.
Travoprost may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure (IOP). If more than one topical ophthalmic drug is being used, the drugs should be administered at least 5 minutes apart.
What are the side effects of the travoprost?
When travoprost administered by the ophthalmic route it may cause some side effects, you should call your doctor immediately if you see any symptoms of allergic reactions for example hives, swelling of the face, lips, tongue, or throat or even difficulty in breathing. You should stop the administration of this medication immediately in case you see these side effects:
- Eye swelling, redness, severe discomfort, crusting or drainage (may be signs of infection)
- Red, swollen, or itchy eyelids
- Increased sensitivity to light
- Vision changes
- Severe burning, stinging, or irritation after using this medicine
Common side effects may include:
- Pain, itching, or redness of the eyes
- Puffy eyelids
- Blurred vision
How to use travoprost ophthalmic?
Before using this medication read all the instructions that are given on the prescription label carefully and follow them. Always use this medication in recommended amounts, do not use it more or less than recommended.
- Every evening, put one drop of this medication in your affected eye and the usual recommended dose is 1 drop in the affected eye. It is important to follow the doctor’s instructions carefully.
- Remove the contact lenses (if you wear), before applying this medication because it can discolor the soft contact lenses due to the presence of a preservative in it. After putting this medication into your eye, wait for at least 15 minutes before wearing your contact lenses.
- Maintain hygiene while using this medication and do not forget to wash your hands before applying this medication.
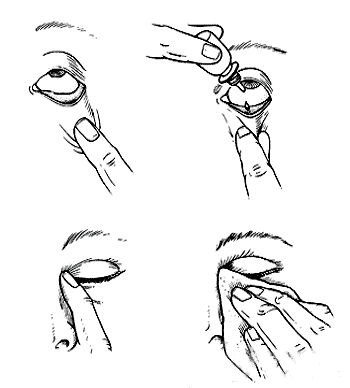
How to apply travoprost eye drops?
To apply travoprost eye drops:
- Tilt your head backward, and create a little pocket in the eye by pulling the lower eyelid downwards. Hold the dropper right above the eye and squeeze out one drop.
- Without blinking or squinting, close your eyes for 2 to 3 minutes. As the liquid can drain into your tear duct, you should gently press your finger for one minute, to the inside corner of the eye in order to prevent the drainage.
- In case your doctor has prescribed you any other eye drops, wait for 5 minutes before using any other medication.
- Maintain hygiene and prevent the contamination of the dropper by not touching the tip of the dropper of the medication container as it can lead to contamination of the medication also. The contamination of the medication can cause serious vision disturbances.
- If the medication contains any kind of particles or impurities in it, or even if the medication seems discolored, avoid using such medicine and buy a new one.
- If you have an eye injury or eye infection, tell your doctor right away.
- If you are using travoprost ophthalmic and you need surgery (such as eye surgery), let your doctor know that you are already using travoprost. Your doctor can stop this medication for a short period of time.
- You should store this medication away from moisture and heat and should be placed at a cool temperature. When the container is not in use, close the container tightly.
Can travopost be used for eyelashes growth?
Travoprost is a new drug which is used to lower the intraocular pressure in patients with ocular hypertension and glaucoma. It is proved more effective than timolol and proved to have equal efficacy as latanoprost in terms of reducing intraocular pressure. However, they showed similar side effects. The eyelashes growth was observed higher in case of travoprost as compared to timolol.
Travoprost increases length and darkness of the eyelash which is known to be associated with eyelash changes that occur at the time of treating ocular hypertension. However, this prostaglandin analogs are not FDA approved for influencing eyelashes growth, not even for the treatment of the hypotrichosis.
Moreover, the efficacy of this agent has also not been evaluated. Therefore you should always consult your doctor before using this medication for eyelashes growth and should not be applied in the infected eye without prior consultation with your doctor.
Can I use travoprost in pregnancy?
The use of travoprost in Australia, during pregnancy is contraindicated. In UK and US, this drug can be used but only when benefit outweighs the risk to the fetus.
It comes under USFDA pregnancy category C which means that there are no sufficient data are given regarding the safety and efficacy of this drug during pregnancy, however the potential benefits can warrant the use of this drug in women who are pregnant, despite the potential risks, it carries.
Therefore pregnant women or even in those women who are trying to get pregnant should practice adequate precautions in order to avoid direct exposure to this medication or even to the container.
Travoprost carcinogenesis, mutagenesis, impairment of fertility
Two-year carcinogenicity studies in mice and rats at subcutaneous doses of 10, 30, or 100 mcg/kg/day did not show any evidence of carcinogenic potential. However, at 100 mcg/kg/day, male rats were only treated for 82 weeks, and the maximum tolerated dose (MTD) was not reached in the mouse study.
The high dose (100 mcg/kg) corresponds to exposure levels over 400 times the human exposure at the maximum recommended human ocular dose (MRHOD) of 0.04 mcg/kg, based on plasma active drug levels.
Travoprost was not mutagenic in the Ames test, mouse micronucleus test or rat chromosome aberration assay. A slight increase in the mutant frequency was observed in one of two mouse lymphoma assays in the presence of rat S-9 activation enzymes.
Travoprost did not affect mating or fertility indices in male or female rats at subcutaneous doses up to 10 mcg/kg/day (250 times the MRHOD of 0.04 mcg/kg/day on a mcg/kg basis). At 10 mcg/kg/day, the mean number of corpora lutea was reduced, and the post-implantation losses were increased. These effects were not observed at 3 mcg/kg/day (75 times the MRHOD).
Can I use travoprost during breastfeeding?
Untill there are safer alternative are present, the use of this medication is not recommended. This drug can excreted into the human milk, therefore one should definitely avoid taking this medication while breastfeeding. The effects of this medication are unknown in the nursing infant, still this drug should be strictly avoided or should not be used without prior consulation to your doctor.
Can I use travopost for hair growth?
As per studies, it has been observed that travoprost when administered on the non daily basis, can induce hair shaft growth more when administered on daily basis. The growth of hair shaft is observed more significant and better when taken on a non-daily basis. It induces growth of hair follicles, and the fact observed was that it is more effective when administered as single treatment as compared to repeated treatment (as seen in 7 days treatment).
Travoprost is known to be useful in reducing the hair loss and heterogenicity in the diameter of the hair shafts as well as it stimulates the growth and density of the hairs. For administration of compositions such as travoprost, one should maintain a time interval in applications, for example, an interval of greater than 24 hours.
Application of the travoprost should be carried out on the non-daily basis, for example, it should be applied every two, three, four, five, six, or seven days.
However, in this study, there were no significant side effects of travoprost was shown, but you should always consult your doctor before using this medication for the purpose of hair growth. Moreover, the excess of this analogs can cause some serious adverse reactions, therefore, avoid using it before consultation with your doctor.
Can travoprost be used for management of open-angle glaucoma and ocular hypertension?
Travoprost is a intraocular pressure (IOP) reducing agent and is a prostaglandin analog, it is significantly used to treat ocular hypertension and glaucoma. It acts by increasing the drainage of aqueous humor via uveoscleral and trabecular outflow channels both.
Travoprost proved to reduce the intraocular pressure and cause a relevant reduction in the mean IOP (i.e. 6.5-9.0mmHg) significantly and safely. Along with the reduction in the IOP, it is known to provide consistent diurnal IOP control that persists up to 84 hours after the administration of the dose.
Travoprost is mainly given for reducing the IOP due to its highly favorable safety profile, and in case the adverse reaction occurs, they are mostly cosmetic in nature such as iris hyperpigmentation and eyelash growth. However, sometimes serious side effects such as iritis and macular edema with travoprost and another prostaglandin analogue.
Travoprost is also coming as a combination with timolol and sold frequently as the fixed combination which should be administered as once daily. The combination is known to reduce the IOP by 7-11.5 mmHg.
However, the monotherapy of travoprost is also considered safe and effective in lowering the IOP, along with the convenience of once-daily dosing. While using, one should always follow the recommendation of the doctor in terms of the doses of such medications.
Can travoprost be taken with tafluprost?
One should generally avoid the concurrent administration of both travoprost and tafluprost. Using multiple prostaglandin analogs and more frequent administration of the prostaglandin (more than recommended) can reduce the main effects of these agents which are lowering intraocular pressure.
When administered concurrently, the combination can exceed the dose-response curve which can also lead to severe effects of intraocular pressure. One can also suffer from other factors such as patient compliance (difficulty in taking multiple medications), disease state (which can flare up if both medications interact with each other).
However, in one study, when two such prostaglandin analogue (bimatoprost and latanoprost) are given to a patient for the treatment of primary open angle glaucoma (non advanced), it showed mean reduction in IOP by 1.8 mmHg in four weeks, which is subsided after discontinuation of the one of the additive prostaglandin analogue (bimatoprost). Therefore the concurrent and frequent administration of travoprost and tafluprost is not recommended by the manufacturers.
Travoprost effects on pigmentation
Travoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid) and eyelashes.
Pigmentation is expected to increase as long as travoprost is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes.
After discontinuation of travoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation.
The long-term effects of increased pigmentation are not known.Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment.
While treatment with travoprost ophthalmic solution 0.004% can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly
Can travoprost be given in patient with macular edema?
Travopost, when given in patient with macular edema, can cause a moderate potential hazard. While treating with an ophthalmic prostaglandin analog (such as travopost), a patient can suffer from macular edema which may include cystoid macular edema also. In patients with aphakia, pseudophakia with a torn posterior lens capsule, the cases of macular edema more frequently reported.
In patients with risk factors for macular edema, for example, posterior uveitis, certain retinal disorders, they are more prone to macular edema. In such patients, the therapy with travopost should be generally avoided or if necessary, it should be used with extreme caution.
Can travoprost provoke bacterial keratitis?
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
Travoprost use with contact lenses
Contact lenses should be removed prior to instillation of travoprost and may be reinserted 15 minutes following its administration.
Can travoprost be given in patients with uveitis?
Some synthetic prostaglandin analogs are bimatoprost, latanoprost, and travoprost. These agents can interfere with the ocular inflammatory responses and also these agents can copy the endogenous prostaglandins.
However, the cases such as uveitis and iritis have been reported rarely. In a patient with active ocular inflammation, the therapy with travopost (and another ophthalmic prostaglandin analogue) should be used with extreme caution.
Can travoprost be given to a patient with liver or renal disease?
In the cornea, the prostaglandin analogs are hydrolyzed by the enzyme esterases and get converted to their active free acid form which is absorbed systemically. It is also get metabolized via fatty acid beta-oxidation by the liver and it is excreted by the kidney.
In patients with impaired renal and hepatic function, these agents have not been studied. Therefore in such patients, the travoprost or other prostaglandin analog therapy should be administered with caution.
“Is it safe to use eye drops after expiration date?“
“What does focalin do? Is focalin considered a narcotic?“